
]ĮņĖįÄĘ¹żŃõ»ÆĒā¼ÓŗĻĪļ(xNa2SO4·yH2O2·zH2O)µÄ×é³ÉæÉĶعżĻĀĮŠŹµŃé²ā¶Ø£ŗ
¢Ł×¼Č·³ĘČ”1.770 0 gѳʷ£¬ÅäÖĘ³É100.00 mLČÜŅŗA”£
¢Ś×¼Č·ĮæČ”25.00 mLČÜŅŗA£¬¼ÓČėŃĪĖįĖį»ÆµÄBaCl2ČÜŅŗÖĮ³ĮµķĶźČ«£¬¹żĀĖ”¢Ļ“µÓ”¢øÉŌļÖĮŗćÖŲ£¬µĆµ½°×É«¹ĢĢå0.582 5 g”£
¢Ū×¼Č·ĮæČ”25.00 mLČÜŅŗA£¬¼ÓŹŹĮæĻ”ĮņĖįĖį»Æŗó£¬ÓĆ0.020 00 mol·L£1 KMnO4ČÜŅŗµĪ¶ØÖĮÖÕµć£¬ĻūŗÄKMnO4ČÜŅŗ25.00 mL”£H2O2ÓėKMnO4·“Ó¦µÄĄė×Ó·½³ĢŹ½ČēĻĀ£ŗ
2MnO £«5H2O2£«6H£«===2Mn2£«£«8H2O£«5O2”ü
£«5H2O2£«6H£«===2Mn2£«£«8H2O£«5O2”ü
(2)ÉĻŹöµĪ¶ØČō²»¼ÓĻ”ĮņĖįĖį»Æ£¬MnO ±»»¹ŌĪŖMnO2£¬ĘäĄė×Ó·½³ĢŹ½ĪŖ________________________________________________________________________”£
±»»¹ŌĪŖMnO2£¬ĘäĄė×Ó·½³ĢŹ½ĪŖ________________________________________________________________________”£
(3)Ķعż¼ĘĖćČ·¶ØѳʷµÄ×é³É(Š“³ö¼ĘĖć¹ż³Ģ)”£
“š°ø””(2)2MnO £«3H2O2===2MnO2”ż£«3O2”ü£«2OH££«2H2O
£«3H2O2===2MnO2”ż£«3O2”ü£«2OH££«2H2O
(3)n(Na2SO4)£½n(BaSO4)£½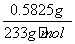
£½2.50”Į10£3 mol
2MnO £«5H2O2£«6H£«===2Mn2£«£«8H2O£«5O2”ü
£«5H2O2£«6H£«===2Mn2£«£«8H2O£«5O2”ü
n(H2O2)£½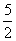 ”Į
”Į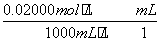 £½1.25”Į10£3 mol
£½1.25”Į10£3 mol
m(Na2SO4)£½142 g·mol£1”Į2.50”Į10£3 mol£½0.355 g
m(H2O2)£½34 g·mol£1”Į1.25”Į10£3 mol£½0.042 5 g
n(H2O)£½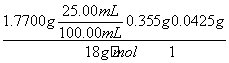 £½2.50”Į10£3 mol
£½2.50”Į10£3 mol
x”Ćy”Ćz£½n(Na2SO4)”Ćn(H2O2)”Ćn(H2O)£½2”Ć1”Ć2
¹ŹŃłĘ·µÄ×é³ÉµÄ»ÆѧŹ½ĪŖ2Na2SO4·H2O2·2H2O”£
½āĪö””(2)H2O2ÓėKMnO4ČÜŅŗ·“Ó¦£¬MnO ±»»¹ŌĪŖMnO2£¬¾ŻµĆŹ§µē×ÓŹŲŗć”¢ÖŹĮæŹŲŗćæÉŠ“³öĄė×Ó·½³ĢŹ½ĪŖ2MnO
±»»¹ŌĪŖMnO2£¬¾ŻµĆŹ§µē×ÓŹŲŗć”¢ÖŹĮæŹŲŗćæÉŠ“³öĄė×Ó·½³ĢŹ½ĪŖ2MnO £«3H2O2===2MnO2”ż£«3O2”ü£«2OH££«2H2O”£
£«3H2O2===2MnO2”ż£«3O2”ü£«2OH££«2H2O”£
(3)25.00 mLČÜŅŗAÖŠŗ¬ÓŠNa2SO4µÄĪļÖŹµÄĮæĪŖn(Na2SO4)£½n(BaSO4)£½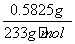 £½2.50”Į10£3 mol”£
£½2.50”Į10£3 mol”£
ŗ¬ÓŠH2O2µÄĪļÖŹµÄĮæĪŖn(H2O2)£½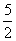 n(KMnO4)£½
n(KMnO4)£½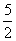 ”Į0.020 00 mol·L£1”Į25.00”Į10£3 L£½1.25”Į10£3 mol”£
”Į0.020 00 mol·L£1”Į25.00”Į10£3 L£½1.25”Į10£3 mol”£
ĖłČ”25.00 mLČÜŅŗAÖŠĖłŗ¬ŃłĘ·ÖŠĖ®µÄÖŹĮæĪŖm(H2O)£½1.770 0 g”Į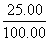 £2.50”Į10£3 mol”Į142 g·mol£1£1.25”Į10£3 mol”Į34 g·mol£1£½0.045 00 g£¬Ōņn(H2O)£½
£2.50”Į10£3 mol”Į142 g·mol£1£1.25”Į10£3 mol”Į34 g·mol£1£½0.045 00 g£¬Ōņn(H2O)£½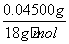 £½2.50”Į10£3 mol”£
£½2.50”Į10£3 mol”£
×ŪÉĻæÉÖŖ£¬x”Ćy”Ćz£½n(Na2SO4)”Ćn(H2O2)”Ćn(H2O)£½2”Ć1”Ć2£¬¹ŹĮņĖįÄĘ¹żŃõ»ÆĒā¼ÓŗĻĪļµÄ»ÆѧŹ½ĪŖ2Na2SO4·H2O2·2H2O”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŌĻĀŠšŹöÖŠ£¬“ķĪóµÄŹĒ(””””)
A£®ÄĘŌ×ÓŗĶĀČŌ×Ó×÷ÓĆÉś³ÉNaClŗó£¬Ęä½į¹¹µÄĪČ¶ØŠŌŌöĒæ
B£®ŌŚĀČ»ÆÄĘÖŠ£¬³żĀČĄė×ÓŗĶÄĘĄė×ӵľ²µēĪüŅż×÷ÓĆĶā£¬»¹“ęŌŚµē×ÓÓėµē×Ó”¢Ō×ÓŗĖÓėŌ×ÓŗĖÖ®¼äµÄÅųā×÷ÓĆ
C£®ČĪŗĪĄė×Ó¼üŌŚŠĪ³ÉµÄ¹ż³ĢÖŠ±Ų¶ØÓŠµē×ӵĵĆÓėŹ§
D£®½šŹōÄĘÓėĀČĘų·“Ӧɜ³ÉĀČ»ÆÄĘŗó£¬ĢåĻµÄÜĮæ½µµĶ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷×éĪļÖŹµÄČŪ”¢·ŠµćøßµĶÖ»Óė·¶µĀ»ŖĮ¦ÓŠ¹ŲµÄŹĒ(””””)
A£®Li”¢Na”¢K”¢Rb
B£®HF”¢HCl”¢HBr”¢HI
C£®LiCl”¢NaCl”¢KCl”¢RbCl
D£®F2”¢Cl2”¢Br2”¢I2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÅŠ¶ĻĻĀĮŠČÜŅŗŌŚ³£ĪĀĻĀµÄĖį”¢¼īŠŌ(ŌŚĄØŗÅÖŠĢī”°ĖįŠŌ”±”¢”°¼īŠŌ”±»ņ”°ÖŠŠŌ”±)”£
(1)ĻąĶ¬ÅØ¶ČµÄHClŗĶNaOHČÜŅŗµČĢå»ż»ģŗĻ(””””)
(2)ĻąĶ¬ÅØ¶ČµÄCH3COOHŗĶNaOHČÜŅŗµČĢå»ż»ģŗĻ(””””)
(3)ĻąĶ¬ÅضČNH3·H2OŗĶHClČÜŅŗµČĢå»ż»ģŗĻ(””””)
(4)pH£½2µÄHClŗĶpH£½12µÄNaOHČÜŅŗµČĢå»ż»ģŗĻ(””””)
(5)pH£½3µÄHClŗĶpH£½10µÄNaOHČÜŅŗµČĢå»ż»ģŗĻ(””””)
(6)pH£½3µÄHClŗĶpH£½12µÄNaOHČÜŅŗµČĢå»ż»ģŗĻ(””””)
(7)pH£½2µÄCH3COOHŗĶpH£½12µÄNaOHČÜŅŗµČĢå»ż»ģŗĻ(””””)
(8)pH£½2µÄHClŗĶpH£½12µÄNH3·H2OµČĢå»ż»ģŗĻ(””””)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
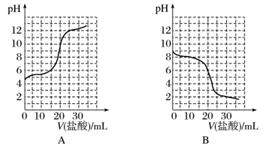
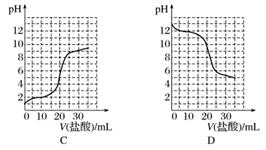
ŅŃÖŖijĪĀ¶ČĻĀCH3COOHµÄµēĄė³£ŹżK£½1.6”Į10£5”£øĆĪĀ¶ČĻĀ£¬Ļņ20 mL 0.01 mol·L£1 CH3COOHČÜŅŗÖŠÖšµĪ¼ÓČė0.01 mol·L£1 KOHČÜŅŗ£¬ĘäpH±ä»ÆĒśĻßČēĶ¼ĖłŹ¾(ŗöĀŌĪĀ¶Č±ä»Æ)”£Ēė»Ų“šĻĀĮŠÓŠ¹ŲĪŹĢā£ŗ
(1)aµćČÜŅŗÖŠc(H£«)ĪŖ________£¬pHŌ¼ĪŖ________”£
(2)a”¢b”¢c”¢dĖĵćÖŠĖ®µÄµēĄė³Ģ¶Č×ī“óµÄŹĒ__________£¬µĪ¶Ø¹ż³ĢÖŠŅĖŃ”ÓĆ____________×÷ÖøŹ¾¼Į£¬µĪ¶ØÖÕµćŌŚ________(Ģī”°cµćŅŌÉĻ”±»ņ”°cµćŅŌĻĀ”±)”£
(3)ČōĻņ20 mLĻ”°±Ė®ÖŠÖšµĪ¼ÓČėµČÅØ¶ČµÄŃĪĖį£¬ŌņĻĀĮŠ±ä»ÆĒ÷ŹĘÕżČ·µÄŹĒ________(Ģī×ÖÄø)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĶ¼±ķŹ¾Ė®ÖŠc(H£«)ŗĶc(OH£)µÄ¹ŲĻµ£¬ĻĀĮŠÅŠ¶Ļ“ķĪóµÄŹĒ (””””)
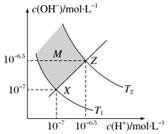
A£®Į½ĢõĒśĻß¼äČĪŅāµć¾łÓŠc(H£«)”Įc(OH£)£½Kw
B£®MĒųÓņÄŚČĪŅāµć¾łÓŠc(H£«)£¼c(OH£)
C£®Ķ¼ÖŠT1£¼T2
D£®XZĻßÉĻČĪŅāµć¾łÓŠpH£½7
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠĮ½ĘæĪĀ¶Č·Ö±šĪŖ15 ”ęŗĶ45 ”ę£¬pH¾łĪŖ1µÄĮņĖįČÜŅŗ£¬ĻĀĮŠÓŠ¹ŲĖµ·Ø²»ÕżČ·µÄŹĒ(””””)
A£®Į½ČÜŅŗÖŠµÄc(OH£)ĻąµČ
B£®Į½ČÜŅŗÖŠµÄc(H£«)ĻąĶ¬
C£®µČĢå»żĮ½ÖÖČÜŅŗÖŠŗĶ¼īµÄÄÜĮ¦ĻąĶ¬
D£®Į½ČÜŅŗÖŠµÄc(H2SO4)»ł±¾ĻąĶ¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĆęŹĒÓŠ¹ŲĪļÖŹµÄ×Ŗ»Æ¹ŲĻµĶ¼(ÓŠŠ©ĪļÖŹŅŃŹ”ĀŌ)”£
ČōAĪŖµ„ÖŹ£¬EŌŚ³£ĪĀĻĀĪŖŅŗĢ壬CµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ78”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ

(1)»³öAµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼________£¬FµÄµē×ÓŹ½ŹĒ________”£
(2)ĻĀĆę¶ŌCĪļÖŹ½į¹¹”¢ŠŌÖŹµÄĶʶĻÖŠ£¬²»ÕżČ·µÄŹĒ________”£
A£®¾ĆÖĆÓŚæÕĘųÖŠ»į±ä³É°×É«
B£®¾ßÓŠĒæŃõ»ÆŠŌ
C£®¾§ĢåÖŠ“ęŌŚĄė×Ó¼üŗĶ¹²¼Ū¼ü
D£®ÓöŹŖČóµÄ×ĻÉ«ŹÆČļŹŌÖ½Ö»ÄÜŹ¹Ęä±äĄ¶É«
(3)ČōCŹĒŗ¬Ńõ»ÆŗĻĪļĒŅŃõĪŖ18OŹ±£¬ŌņCÓėD·“Ó¦ĖłµĆ²śĪļµÄĦ¶ūÖŹĮæ·Ö±šĪŖ__________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Na2CO3¹ĢĢå·ŪÄ©ÖŠ»ģÓŠÉŁĮæNaHCO3£¬ÓĆŹ²Ć“·½·Ø³żŌÓ£æNa2CO3ČÜŅŗÖŠ»ģÓŠÉŁĮæNaHCO3£¬ÓĆŹ²Ć“·½·Ø³żŌÓ£æNaHCO3ČÜŅŗÖŠ»ģÓŠÉŁĮæNa2CO3£¬ÓĆŹ²Ć“·½·Ø³żŌÓ£æ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com