
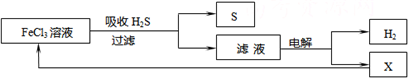
| A������H2S�����ӷ���ʽΪ��2Fe3++H2S=2Fe2++S��+2H+ |
| B���������е�������Ӧ��ҪΪ��2Cl--2e-=Cl2�� |
| C���ù�������������ɫ��ѧ˼�� |
| D��ʵ���ҿ��õ�ȼ���ȼ�յķ�������H2S��Ⱦ |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������H2S�����ӷ���ʽΪ��2Fe3++H2S�T2Fe2++S��+2H+ | B���������е�������Ӧ��ҪΪ��2Cl--2e-�TCl2�� | C��������X�ڸù��������п�ѭ������ | D��ʵ���ҿ��õ�ȼ���ȼ�յķ�������H2S��Ⱦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
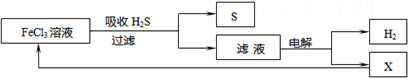
��ͼ��ij�о�С����õ�ⷨ����ʯ�����ƹ����в����Ĵ���H2S�����Ĺ������̡��÷�����H2S�������ʴ�99%���ϣ�������ȡH2��S������˵����ȷ���� ( )

A������H2S�����ӷ���ʽΪ��Fe3��+H2S = Fe2��+S��+2H��
B���������е�������Ӧ��ҪΪ��2Cl����2e�� = Cl2��
C���ù�������������ɫ��ѧ˼��
D��ʵ���ҿ��õ�ȼ���ȼ�յķ�������H2S��Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ij�о�С����õ�ⷨ����ʯ�����ƹ����в����Ĵ���H2S�����Ĺ������̡��÷�����H2S�������ʴ�99%���ϣ�������ȡH2��S������˵����ȷ���� ( )
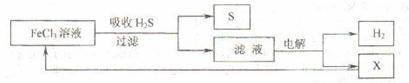
A������H2S�����ӷ���ʽΪ��Fe3��+H2S = Fe2��+S��+2H��
B���������е�������Ӧ��ҪΪ��2Cl����2e�� = Cl2��
C���ù�������������ɫ��ѧ˼��
D��ʵ���ҿ��õ�ȼ���ȼ�յķ�������H2S��Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ����ֶ�����������ѧ����ģ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
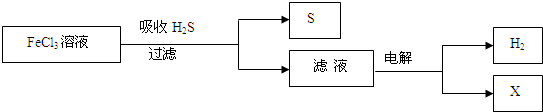
��ͼ��ij�о�С����õ�ⷨ����ʯ�����ƹ����в����Ĵ���H2S�����Ĺ������̡��÷�����H2S�������ʴ�99%���ϣ�������ȡH2��S������˵����ȷ����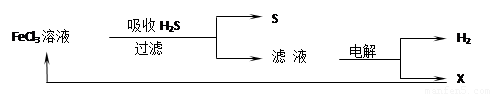
A������H2S�����ӷ���ʽΪ��2Fe3++H2S��2Fe2++S��+2H+
B���������е�������Ӧ��ҪΪ��2Cl����2e����Cl2
C���ù�������������ɫ��ѧ˼��
D��ʵ���ҿ��õ�ȼ���ȼ�յķ�������H2S��Ⱦ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com