
| ���� | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| ��ʼ������pH | 1.8 | 6.3 | 5.2 |
| ��ȫ������pH | 3.0 | 8.3 | 6.7 |
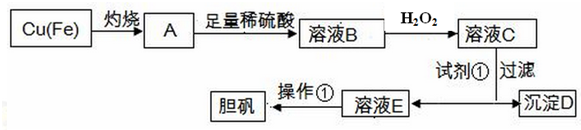
���� �������������Ǽ���Ũ���ᣬ��ͭ�ܽ�Ϊͭ���ӣ�ͬʱ���Ļ������Զ��ۻ����۽�����Һ���ڢڲ�����H2O2��Һ��������������Ϊ���������ڢ۲��ټ���CuO��pH��3.7��5.2֮�䣬���˳�ȥ������������������ͭ����������Һ��Ϊ�˷�ֹͭ�����ڼ���Ũ��ʱˮ�⣬�������������ȣ�����ˮ�⣮����ҺŨ����һ���̶ȣ���ȴ���ɽᾧ�����������˼��ò�Ʒ��
��1��H2O2��Fe2+����������ԭ��Ӧ��
��2�������ӵļ��鷽���ǣ�����Һ�м���KSCN��Һ���۲���������Һ���ɫ��˵����Һ�д���Fe3+����û�б�ɺ�ɫ��֤����Һ��û�������ӣ�
��3�������Լ��ٵ�����Һ��pHֵ��ʹ����������ȫ��������������ͭ��������
��4������Ksp[Fe��OH��3]����Һ�����������ӵ�Ũ�ȼ���c��Fe3+����Qc��Ksp[Cu��OH��2]����Դ�С�жϣ�
��5�������ٵ���Ҫ����Ϊ����Ũ������ȴ�ᾧ�Եõ�����ͭ���壻
��6���١�ǡ�÷�Ӧ��ʱ����Һ�е�I2ǡ����Na2S2O3��ȫ��Ӧ��
�ڸ��ݷ�Ӧ�еĸ�����֮��Ĺ�ϵ�ɼ����Cu2+���ӵ����ʵ������ٸ���c=$\frac{n}{V}$����Ũ�ȣ�
��� �⣺��1��H2O2��Fe2+����������ԭ��Ӧ�����ӷ���ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��
�ʴ�Ϊ��2Fe2++H2O2+2H+=2Fe3++2H2O��
��2��������Һ���Ƿ���Fe3+�ķ���Ϊ������Һ�м���KSCN��Һ��Ȼ��۲���������Һ���ɫ��˵����Һ�д���Fe3+������û�������ӣ�
�ʴ�Ϊ������Һ�м���KSCN��Һ��Ȼ��۲���������Һ���ɫ��˵����Һ�д���Fe3+������û�������ӣ�
��3���ڢ۲�Ŀ���ǵ���pHֵ��ʹ�ü������ʣ������������µ����ʣ���ѡ��CuO��Cu��OH��2��Cu2��OH��2CO3��ʹPH����������������ȫ��������������ͭ���������ʴ�Ϊ��3.7-5.2��CuO��Cu��OH��2��Cu2��OH��2CO3
��4����֪��Һ��pH=4����c��H+��=10-4mol/L��c��OH-��=10-10mol/L��Ksp[Fe��OH��3]=4.0��10-38=c��Fe3+����c3��OH-��������c��Fe3+��=4.0��10-8mol/L��
��֪��Һ�����������ӵ�Ũ��Ϊ10-10mol/L����Qc=c��Cu2+����c2��OH-��=1����10-10��2=10-20��Ksp[Cu��OH��2]=2.2��10-20������û��������ͭ�������ɣ�
�ʴ𰸣�4.0��10-8���ޣ�
��5�������ٵ���Ҫ����Ϊ����Ũ������ȴ�ᾧ�Եõ�����ͭ���壬
�ʴ�Ϊ������Ũ������ȴ�ᾧ��
��6���١�ǡ�÷�Ӧ��ʱ����Һ�е�I2ǡ����Na2S2O3��ȫ��Ӧ����ʱ��Һ����ɫ�仯Ϊ��ɫ��Ϊ��ɫ��
�ʴ�Ϊ����ɫ��Ϊ��ɫ��
�ڸ��ݷ�Ӧ2Cu2++4I-�T2CuI��+I2��2S2O32-+I2�TS4O62-+2I-��֪��2Cu2+��I2��2S2O32-������Cu2+���ӵ����ʵ�����S2O32-�����ʵ�����ȣ�ΪcV2��10-3 mol������c=$\frac{n}{V}$��֪��Cu2+���ӵ����ʵ���Ũ��Ϊ$\frac{c{V}_{2}}{{V}_{1}}$mol/L��
�ʴ�Ϊ��$\frac{c{V}_{2}}{{V}_{1}}$��
���� ���⿼�����ʵ��Ʊ��������IJⶨ��������ѧ��ʵ�������ͷ��������Ŀ��飬Ϊ�߿��������ͣ�ע�⣨4���⣬���ù�ϵʽ�������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʯ�� | B�� | �������ƣ�Na2SO3�� | C�� | ���� | D�� | ����������FeSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���������� | �� �� | |
| A | ������ˮ��ʪ��pH��ֽ����Һ��pH | һ����ʹ�ⶨ���ƫ�� |
| B | ���������Һ�У��������� | ������֤S��������ǿ��Si |
| C | ��SO2ͨ����ˮ�У���ˮ��ɫ | SO2����Ư���� |
| D | ��������FeBr2��FeCl2��Һ�У�����������ˮ���ټ�CCl4��ȡ��Һ | ��ȥFeCl2��Һ�е�FeBr2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| HCN | HF | CH3COOH | HNO2 | |
| Ka | 6.2��10-10 | 6.8��10-4 | 1.8��10-5 | 6.4��10-6 |
| A�� | HCN | B�� | CHSCOOH | C�� | HF | D�� | HNOp |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3COOH��aq��+NaOH��aq���TCH3COONa��aq��+H2O��l����H=-57.3kJ/mol | |
| B�� | KOH��aq��+$\frac{1}{2}$H2SO4��aq��=$\frac{1}{2}$K2SO4��aq��+H2O��l����H=+57.3kJ/mol | |
| C�� | C8H18��l��+$\frac{25}{2}$ O2 ��g��=8CO2 ��g��+9H2O��g����H=-5518kJ/mol | |
| D�� | 2C8H18��g��+25O2 ��g��=16CO2 ��g��+18H2O��1����H=-11036kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
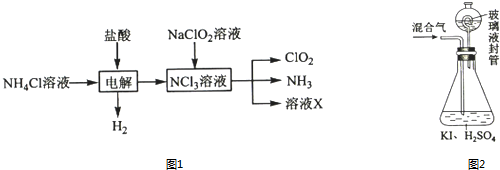
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
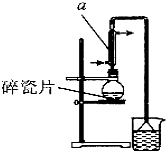 ������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ� 1-�嶡��ķ�Ӧ��ʵ��װ�����£�
������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ� 1-�嶡��ķ�Ӧ��ʵ��װ�����£�| �۵�/�� | �е�/�� | �ܶ�/g•cm-3 | |
| ������ | -89.53 | 117.25 | 0.81 |
| 1-�嶡�� | -112.4 | 101.6 | 1.28 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 40.5% | B�� | 60.6% | C�� | 81.0% | D�� | 100% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
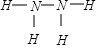 ����֪Һ̬�µı�ȼ����Ϊ-622kJ/mol��д������ȼ�շ������Ȼ�ѧ����ʽ��N2H4��l��+O2��g��=N2��g��+2H2O��l������H=-622KJ/mol��
����֪Һ̬�µı�ȼ����Ϊ-622kJ/mol��д������ȼ�շ������Ȼ�ѧ����ʽ��N2H4��l��+O2��g��=N2��g��+2H2O��l������H=-622KJ/mol���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com