
W��Q��A��B��X��Y��ZΪԭ��������������Ķ�����Ԫ�أ���֪W��X��ͬһ���壻Q�������Ӻ�A�������Ӿ�����ͬ�ĵ��Ӳ�ṹ������Ԫ�صĵ��ʷ�Ӧ������һ�ֵ���ɫ�Ĺ���F��A��B��Z����Ԫ�ص�ԭ������㹲��11�����ӣ�����3��Ԫ�ص�����������ˮ�����������ܷ�����Ӧ�����κ�ˮ��XԪ�ص������������ȴ�����������4��YԪ�ص�L�����������K��M�������Ӳ��ϵĵ�����֮�͡�
��1��WԪ�������ڱ��е�λ���� ��
����ɫ����F�к��еĻ�ѧ���� ��
��2��A��Y��Ԫ�ؿ��γɻ�����õ���ʽ��ʾ�˻�������γɹ��̣�
��
��3��д��A��B��Ԫ�ص�����������ˮ�������Ӧ�����ӷ���ʽ��
��
��4��A��B��Y��Z����Ԫ�صļ����ӵ����Ӱ뾶�ɴ�С��˳��Ϊ�� �����������������ӷ��ű�ʾ��
��5��X��Y��Z����Ԫ�ص��⻯����ȶ�����ǿ������˳��Ϊ�� �����û�ѧʽ��ʾ��
��ÿ��2�֣���12�֣�
��1���ڶ����ڡ���IVA�壨��ȫ���÷֣� �� ���Ӽ����Ǽ��Թ��ۼ�����ȫ���÷֣�
��2��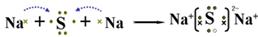
��3��Al(OH)3+ OH- = AlO2-+2H2O
��4��S2����Cl����Na+��Al3+
��5��HCl��H2S��SiH4
�����������������Q�������Ӻ�A�������Ӿ�����ͬ�ĵ��Ӳ�ṹ������Ԫ�صĵ��ʷ�Ӧ������һ�ֵ���ɫ�Ĺ���F��FΪNa2O2��QΪO��AΪNa������A��B��Z����Ԫ�ص�ԭ������㹲��11�����ӣ�����3��Ԫ�ص�����������ˮ�����������ܷ�����Ӧ�����κ�ˮ��BΪAl��ZΪCl������XԪ�ص������������ȴ�����������4��XΪSi������W��X��ͬһ���壬WΪC������YԪ�ص�L�����������K��M�������Ӳ��ϵĵ�����֮�ͣ�YΪS��
��1��WΪC���������ڱ��е�λ���ǵڶ����ڡ���IVA�塣
����ɫ����FΪNa2O2�����еĻ�ѧ�������Ӽ����Ǽ��Թ��ۼ���
��2��A��Y��Ԫ���γɻ�����ΪNa2S������ʽ��ʾ�˻�������γɹ���Ϊ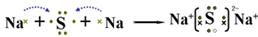 ��
��
��3��A��B��Ԫ�ص�����������ˮ����ֱ�ΪNaOH��Al(OH)3����Ӧ�����ӷ���ʽΪAl(OH)3+ OH- = AlO2-+2H2O��
��4��A��B��Y��Z����Ԫ�صļ����ӵ����ӷֱ�ΪNa+��Al3+��S2����Cl�������Ӳ���Խ�࣬�뾶Խ������ͬ�����Ų������ӣ��˵����Խ��뾶ԽС�������Ӱ뾶�ɴ�С��˳��ΪS2����Cl����Na+��Al3+��
��5��X��Y��Z����Ԫ�ص��⻯��ֱ�ΪSiH4��H2S��HCl��Ԫ�صķǽ�����Խǿ����Ӧ���⻯����ȶ���Խǿ�����⻯����ȶ�����ǿ������˳��ΪHCl��H2S��SiH4 ��
���㣺ԭ�ӽṹ��Ԫ�������ʵĹ�ϵ ����ʽ ��ѧ����ʽ����д
���������⿼��ԭ�ӵķ��ţ�����ʽ����ѧ����ʽ����д���ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��������ѧ�߶���һ���¿����ص�ࣩ��ѧ�Ծ����������� ���ͣ�������
(6��)����ͼ��ʾ���رջ���K����A�г���1molX��1molY����B�г���2molX��2molY����ʼʱVA=VB=aL������ͬ�¶Ⱥ��д������ڵ������£��������и��Է���������Ӧ��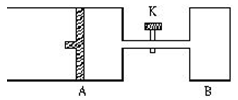
X+Y 2Z+2W+Q����H��0��X��Y��Z��W��Ϊ���壩���ﵽƽ��ʱ��VA=1.2aL���Իش�
2Z+2W+Q����H��0��X��Y��Z��W��Ϊ���壩���ﵽƽ��ʱ��VA=1.2aL���Իش�
��1��A��X��ת���ʦ�A=________��
��2��A��B��Xת���ʵĹ�ϵ����A________��B�����������=����������
��3������K��һ��ʱ����ִﵽƽ��ʱ��A�����Ϊ________L����ͨ��������������ƣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��ɽ��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
W��Q��A��B��X��Y��ZΪԭ��������������Ķ�����Ԫ�أ���֪W��X��ͬһ���壻Q�������Ӻ�A�������Ӿ�����ͬ�ĵ��Ӳ�ṹ������Ԫ�صĵ��ʷ�Ӧ������һ�ֵ���ɫ�Ĺ���F��A��B��Z����Ԫ�ص�ԭ������㹲��11�����ӣ�����3��Ԫ�ص�����������ˮ�����������ܷ�����Ӧ�����κ�ˮ��XԪ�ص������������ȴ�����������4��YԪ�ص�L�����������K��M�������Ӳ��ϵĵ�����֮�͡�
��1��WԪ�������ڱ��е�λ���� ��
����ɫ����F�к��еĻ�ѧ���� ��
��2��A��Y��Ԫ�ؿ��γɻ�����õ���ʽ��ʾ�˻�������γɹ��̣�
��
��3��д��A��B��Ԫ�ص�����������ˮ�������Ӧ�����ӷ���ʽ��
��
��4��A��B��Y��Z����Ԫ�صļ����ӵ����Ӱ뾶�ɴ�С��˳��Ϊ�� �����������������ӷ��ű�ʾ��
��5��X��Y��Z����Ԫ�ص��⻯����ȶ�����ǿ������˳��Ϊ�� �����û�ѧʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
W��Q��A��B��X��Y��ZΪԭ��������������Ķ�����Ԫ�أ���֪W��X��ͬһ���壻Q�������Ӻ�A�������Ӿ�����ͬ�ĵ��Ӳ�ṹ������Ԫ�صĵ��ʷ�Ӧ������һ�ֵ���ɫ�Ĺ���F��A��B��Z����Ԫ�ص�ԭ������㹲��11�����ӣ�����3��Ԫ�ص�����������ˮ�����������ܷ�����Ӧ�����κ�ˮ��XԪ�ص������������ȴ�����������4��YԪ�ص�L�����������K��M�������Ӳ��ϵĵ�����֮�͡�
��1��WԪ�������ڱ��е�λ���� ��
����ɫ����F�к��еĻ�ѧ���� ��
��2��A��Y��Ԫ�ؿ��γɻ�����õ���ʽ��ʾ�˻�������γɹ��̣�
��
��3��д��A��B��Ԫ�ص�����������ˮ�������Ӧ�����ӷ���ʽ��
��
��4��A��B��Y��Z����Ԫ�صļ����ӵ����Ӱ뾶�ɴ�С��˳��Ϊ�� ��������������
���ӷ��ű�ʾ��
��5��X��Y��Z����Ԫ�ص��⻯����ȶ�����ǿ������˳��Ϊ�� ������
��ѧʽ��ʾ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com