
 Sn����������ʹ�õ�Ԫ��֮һ�����γ�SnCl2��SnCl4�����Ȼ��SnCl2������Ϊ��ɫ���壬����һά��״�ľۺϽṹ����̬ʱ�Ե�������ʽ���ڣ���SnCl4������Ϊ��ɫҺ�壮�����ͻ�����Sn������ͬ�������壬�����ľ���ṹ��Snԭ�ӵ���λ��Ϊ4��6�������ľ���ṹ����ʯ�ľ���ṹ���ƣ��������ܶȴ��ڻ������ܶȣ�
Sn����������ʹ�õ�Ԫ��֮һ�����γ�SnCl2��SnCl4�����Ȼ��SnCl2������Ϊ��ɫ���壬����һά��״�ľۺϽṹ����̬ʱ�Ե�������ʽ���ڣ���SnCl4������Ϊ��ɫҺ�壮�����ͻ�����Sn������ͬ�������壬�����ľ���ṹ��Snԭ�ӵ���λ��Ϊ4��6�������ľ���ṹ����ʯ�ľ���ṹ���ƣ��������ܶȴ��ڻ������ܶȣ�| M |
| V |
| 4+4 |
| 2 |
| 1 |
| 2 |
| 1 |
| 8 |
| 952 |
| NA(a��10-10)3 |
| 952 |
| NA(a��10-10)3 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A��X��YΪͬһ������ |
| B��X��Y�������ӳɺ�����ͬһ������ |
| C��X��Y�����Է����Ӿ۷�Ӧ |
| D��X��Y����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| HBr |
| ���� |
| ����a |
| NaOH��Һ |
| �� |
| ����B |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ڣ��ۣ��ܣ��� |
| B���ܶȣ��ڣ��ۣ��ܣ��� |
| C���������ڣ��ۣ��ܣ��� |
| D��Hԭ�Ӹ������٣��ۣ��ڣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� ��NaOH��Һ����ڲ���ƿ�� |
B�� ������Һ��NH4+�Ĵ��� |
C��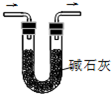 ����SO2���� |
D�� �ռ�HCl���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com