
��10�֣��������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����C02�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӡ�
(1)���й���C02����;����ȷ���� ������ţ���
A��������̼������ˮ������������̼��������
B�����������̼�׳Ƹɱ����������˹�����
C��������̼�����������Ϊ�����˿�ȼ����Ż��
D�����ٽ������̼����ȡ�ܼ����ڴ���Ȼ������ȡ�;����������
E��������̼������Ϊ���ʣ����ũ����IJ���
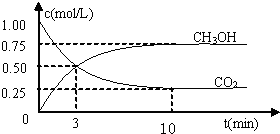
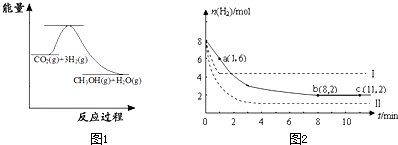
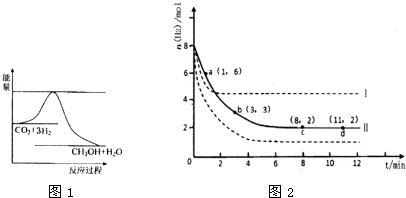
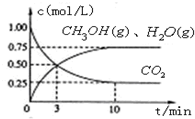
(2)Ŀǰ��ҵ����һ�ַ�������C02�������״���Ϊ̽���䷴Ӧԭ�����ֽ�������ʵ�飬�����Ϊ1L���ܱ������У�����1 mol CO2��3 mol H2��һ�������·�����Ӧ��C02(g)+
3 H2(g) ![]() CH30H(g)+H20(g) ��H=-49.0kJ��m01-1�����C02��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH30H(g)+H20(g) ��H=-49.0kJ��m01-1�����C02��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ���ʣ�v(H��)= ��
�ڸ÷�Ӧ��ƽ�ⳣ��Ϊ ��
(3)��KHC03��ҺΪ����ʣ��õ��ķ���Ҳ���Խ�C02��ԭΪ�״�����д�����ʱ���������ӷ���ʽ��
(4)���ʯ��ʯīϩ������̼��̼�ļ���ͬ�������塣�Ӽ������ͷ������ǵ��ȶ�����ǿ������˳��Ϊ
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ��ע
�������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ��ע| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
| c(CH3OH)?c(H2O) |
| c(CO2)?c3(H2) |
| 16 |
| 3 |
| 16 |
| 3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| c(CH3OH)��c(H2O) |
| c(CO2)��c3(H2) |
| c(CH3OH)��c(H2O) |
| c(CO2)��c3(H2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 | 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com