
ŅŃÖŖČČ»Æѧ·½³ĢŹ½£ŗ
(1)2H2O(l)===2H2(g)£«O2(g)
¦¤H£½£«571.6 kJ”¤mol£1
(2)2H2(g)£«O2(g)===2H2O(g)
¦¤H£½£483.6 kJ”¤mol£1
µ±1 gŅŗĢ¬Ė®±äĪŖĘųĢ¬Ė®Ź±£¬¶ŌĘäČČĮæ±ä»ÆÓŠĻĀĮŠĆčŹö£ŗ¢Ł·Å³ö£»¢ŚĪüŹÕ£»¢Ū2.44 kJ£»¢Ü4.88 kJ£»¢Ż88 kJ”£ĘäÖŠÕżČ·µÄŹĒ(””””)””””””””””””””””””””””””””””””””””””””
A£®¢ŚŗĶ¢Ż B£®¢ŁŗĶ¢Ū C£®¢ŚŗĶ¢Ü D£®¢ŚŗĶ¢Ū

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½üŅ»Š©ÄźĄ“£¬µĶĢ¼Éś»ī½«Öš½„³ÉĪŖŹ±“śµÄŠĀŹ±ÉŠ”£ĻĀĮŠ²»·ūŗĻµĶĢ¼Éś»īøÅÄīµÄŹĒ A£®°Ń°×³ćµĘ»»³É½ŚÄÜµĘ B£®×Ō±ø¹ŗĪļ²¼“ü£¬²»ÓĆĖÜĮĻ·½±ć“ü
C£®ŹÕ¼ÆĮÜŌ”Ė®ÓĆÓŚĶĻµŲ”¢½½»Ø D£®øųĘū³µ°²×°Ī²Ęų“¦ĄķĘ÷
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ(””””)
A£®·Ö×Ó×é³ÉĻą²ī1øö»ņČōøÉøöCH2Ō×ÓĶŵÄĪļÖŹ»„³ĘĪŖĶ¬ĻµĪļ
B£®Ļą¶Ō·Ö×ÓÖŹĮæĻąĶ¬µÄÓŠ»śĪļŹĒĶ¬·ÖŅģ¹¹Ģå
C£®Ģ¼Ō×ÓÖ®¼äÖ»ŅŌµ„¼üĻą½įŗĻµÄĮ“ĢžĪŖĶéĢž
D£®·Ö×ÓŹ½ĻąĶ¬ÓŠ»śĪļŅ»¶ØŹĒĶ¬Ņ»ÖÖĪļÖŹ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
1 molijĢžAŗĶ1 mol±½ĶźČ«Č¼ÉÕ£¬ĢžA±Č±½¶ąĻūŗÄ1 mol O2£¬ČōA·Ö×Ó½į¹¹ÖŠĪŽÖ§Į“»ņ²ąĮ“£¬Ōņ£ŗ
(1)ČōAĪŖ»·×“»ÆŗĻĪļ£¬ĖüÄÜÓėµČĪļÖŹµÄĮæµÄBr2·¢Éś¼Ó³É·“Ó¦£¬ŌņAµÄ½į¹¹¼ņŹ½ĪŖ________________________________________________________________________£»
(2)ČōAĪŖĮ“דĻ©Ģž£¬1 mol A×ī¶ąæÉŗĶ2 mol Br2·¢Éś¼Ó³É·“Ó¦ĒŅAÓėµČĪļÖŹµÄĮæµÄBr2¼Ó³ÉŗóµÄæÉÄܲśĪļÖ»ÓŠ2ÖÖ£¬ŌņAµÄ½į¹¹¼ņŹ½ĪŖ_____________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚĻĀĮŠø÷Ėµ·ØÖŠ£¬ÕżČ·µÄŹĒ(””””)
A£®¦¤H>0±ķŹ¾·ÅČČ·“Ó¦£¬¦¤H<0±ķŹ¾ĪüČČ·“Ó¦
B£®ČČ»Æѧ·½³ĢŹ½ÖŠµÄ»Æѧ¼ĘĮæŹżÖ»±ķŹ¾ĪļÖŹµÄĮ棬æÉŅŌŹĒ·ÖŹż
C£®1 mol H2SO4Óė1 mol Ba(OH)2·“Ӧɜ³ÉBaSO4³ĮµķŹ±·Å³öµÄČČ½Š×öÖŠŗĶČČ
D£®1 mol H2Óė0.5 mol O2·“Ó¦·Å³öµÄČČ¾ĶŹĒH2µÄČ¼ÉÕČČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
NH3(g)Č¼ÉյIJśĪļŹĒNO2(g)ŗĶH2O(g)”£ŅŃÖŖ·“Ó¦ÓŠ£ŗ
(1)H2(g)£«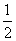 O2(g)===H2O(g) ¦¤H£½£241.8 kJ”¤mol£1
O2(g)===H2O(g) ¦¤H£½£241.8 kJ”¤mol£1
(2)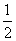 N2(g)£«O2(g)===NO2(g)”” ¦¤H£½£«33.9 kJ”¤mol£1
N2(g)£«O2(g)===NO2(g)”” ¦¤H£½£«33.9 kJ”¤mol£1
(3)NH3(g)===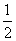 N2(g)£«
N2(g)£«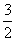 H2(g) ¦¤H£½£«46.0 kJ”¤mol£1
H2(g) ¦¤H£½£«46.0 kJ”¤mol£1
ĻĀĮŠ¹ŲÓŚNH3(g)Č¼ÉÕµÄČČ»Æѧ·½³ĢŹ½µÄŹéŠ“ÕżČ·µÄŹĒ(””””)
A£®NH3(g)£«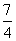 O2(g)===NO2(g)£«
O2(g)===NO2(g)£«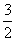 H2O(g) ¦¤H£½£282.8 kJ”¤mol£1
H2O(g) ¦¤H£½£282.8 kJ”¤mol£1
B£®NH3(g)£«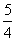 O2(g)===NO2(g)£«
O2(g)===NO2(g)£«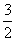 H2O(g) ¦¤H£½£161.9 kJ”¤mol£1
H2O(g) ¦¤H£½£161.9 kJ”¤mol£1
C£®NH3(g)£«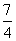 O2(g)===NO2(g)£«
O2(g)===NO2(g)£«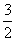 H2O(g) ¦¤H£½£161.9 kJ”¤mol£1
H2O(g) ¦¤H£½£161.9 kJ”¤mol£1
D£®NH3(g)£«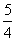 O2(g)===NO2(g)£«
O2(g)===NO2(g)£«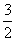 H2O(g) ¦¤H£½£282.8 kJ”¤mol£1
H2O(g) ¦¤H£½£282.8 kJ”¤mol£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŚĀåĶŠĘ·ŌŚŗĻ³É”¢Ņ½Ņ©”¢Č¾ĮĻµČ¹¤ŅµÖŠÓŠ¹ć·ŗÓĆĶ¾£¬Ęä½į¹¹Ź½ČēĶ¼ĖłŹ¾”£½«¼×Č©Ė®ČÜŅŗÓė°±Ė®»ģŗĻÕō·¢æÉÖʵĆĪŚĀåĶŠĘ·”£ČōŌĮĻĶźČ«·“Ӧɜ³ÉĪŚĀåĶŠĘ·£¬Ōņ¼×Č©Óė°±µÄĪļÖŹµÄĮæÖ®±ČĪŖ£Ø £©
A£®1”Ć1 B£®2”Ć3 C£®3”Ć2 D£®2”Ć1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠŠšŹöÕżČ·µÄŹĒ£Ø £©
A. ŅŅ“¼ŹĒ×ī¼ņµ„µÄ±„ŗĶŅ»ŌŖ“¼
B. °±»łĖįŹōÓŚøß·Ö×Ó»ÆŗĻĪļ
C. C3H6ŗĶC4H8Ņ»¶ØŹōÓŚĶ¬ĻµĪļ
D. ĘĻĢŃĢĒÓėŠĀÖʵÄCu(OH)2¹²ČČ£¬³öĻÖשŗģÉ«»ė×Ē£¬±ķĆ÷ĘĻĢŃĢĒ¾ßÓŠ»¹ŌŠŌ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)½«ŠæʬŗĶŅųʬ½žČėĻ”ĮņĖįÖŠ×é³ÉŌµē³Ų£¬Į½µē¼«¼äĮ¬½ÓŅ»øöµēĮ÷¼Ę”£
ŠæʬÉĻ·¢ÉśµÄµē¼«·“Ó¦£ŗ___________________________________________________£»
ŅųʬÉĻ·¢ÉśµÄµē¼«·“Ó¦£ŗ_________________________________________________”£
(2)ČōøƵē³ŲÖŠĮ½µē¼«µÄ×ÜÖŹĮæĪŖ60 g£¬¹¤×÷Ņ»¶ĪŹ±¼äŗó£¬Č”³öŠæʬŗĶŅųʬĻ“¾»øÉŌļŗó³ĘÖŲ£¬×ÜÖŹĮæĪŖ47 g£¬ŹŌ¼ĘĖć£ŗ
¢Ł²śÉśĒāĘųµÄĢå»ż(±ź×¼×“æö)£»
¢ŚĶعżµ¼ĻߵĵēĮ攣(ŅŃÖŖNA£½6.02”Į1023 mol£1£¬µē×ÓµēĮæĪŖ1.60”Į10£19C)
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com