
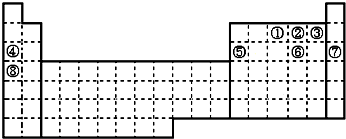
 ���û�ѧʽ��ʾ����ͬ����д���ں���10�����ӵĺ��صĻ�ѧ����818O��
���û�ѧʽ��ʾ����ͬ����д���ں���10�����ӵĺ��صĻ�ѧ����818O������ ��Ԫ�ص�λ�ÿ�֪���١���ֱ�ΪN��O��F��Na��Al��S��Ar��K��
��1��S�����ӽṹ����3�����Ӳ㣬����������Ϊ8���ں���10�����ӵĺ��أ�������Ϊ18��
��2��������ͬ�����Ų������ӣ�ԭ������������Ӱ뾶С��
��3���ǽ�����Խǿ����̬�⻯��Խ�ȶ���
��4����������������NO��ˮ��
��5��Al����������������ԣ�Al��KOH��Ӧ����ƫ����غ�������
��6��1molNaȼ�����ɹ������ƣ��ų�����Ϊ255.5kJ���Դ���д�Ȼ�ѧ����ʽ��
��� �⣺��Ԫ�ص�λ�ÿ�֪���١���ֱ�ΪN��O��F��Na��Al��S��Ar��K��
��1��S�����ӽṹ����3�����Ӳ㣬����������Ϊ8�����ӽṹʾ��ͼΪ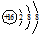
�ʴ�Ϊ��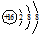
��2��������ͬ�����Ų������ӣ�ԭ������������Ӱ뾶С�������Ӱ뾶Ϊr��O2-����r��F-����r��Na+����r��Al3+����
�ʴ�Ϊ��r��O2-����r��F-����r��Na+����r��Al3+����
��3���ǽ�����Խǿ����̬�⻯��Խ�ȶ����⻯����ȶ�����ǿ������˳��ΪHF��H2O���ʴ�Ϊ��HF��H2O��
��4����������������NO��ˮ����ѧ��ӦΪ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O���ʴ�Ϊ��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
��5��Al����������������ԣ�Al��KOH��Ӧ����ƫ����غ����������ӷ�ӦΪ2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��Al��2Al+2OH-+2H2O=2AlO2-+3H2����
��6��1molNaȼ�����ɹ������ƣ��ų�����Ϊ255.5kJ���Ȼ�ѧ����ʽΪ2Na��s��+O2��g��=Na2O2��s����H=-511kJ•mol-1���ʴ�Ϊ��2Na��s��+O2��g��=Na2O2��s����H=-511kJ•mol-1��
���� ���⿼��λ�á��ṹ�����ʣ�Ϊ��Ƶ���㣬����Ԫ�ص�λ�á�ԭ�ӽṹ�ƶ�Ԫ�ص�Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע��Ԫ�������ɵ�Ӧ�ã���Ŀ�ѶȲ���


 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HF��HCl��H2S��PH3���ȶ���������ǿ | |
| B�� | Na��Mg��Al��Si�Ļ�ԭ������ǿ | |
| C�� | O��S��Na��K��ԭ�Ӱ뾶�������� | |
| D�� | KOH��Ca��OH��2��Mg��OH��2��Al��OH��3�ļ�������ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

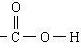
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CHICH2 CH3 | B�� | CH3OH | C�� | ��CH3��3COH | D�� | ��CH3��3C-CH2C1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ס��졢�� | B�� | �졢�ڡ��� | C�� | �졢�졢�� | D�� | �ס��ڡ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Na2CO3��Һ��ͨ����CO2��CO32-+CO2+H2O�T2HCO3- | |
| B�� | �μ�ص�������CO32-+CaSO4�TCaCO3+SO42- | |
| C�� | ��ĭ�������Ӧԭ����2 Al3++3CO32-+3H2O�T2Al��OH��3��+3CO2�� | |
| D�� | ���CuSO4��Һ��2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+4H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Ǵ�����й̶����۷е� | |
| B�� | ���Ϳ�����ʯ�͵ķ���õ� | |
| C�� | ���þ��ú����Ƿ�ֲ����ж�ʳ�������Ƿ�������� | |
| D�� | �ѻ����Ϳ�����Ϊ��ˮ����ȡ�����ȡ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��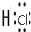 ��
�� ��
��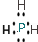 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ڢ� | C�� | �٢ۢ� | D�� | �ڢۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com