
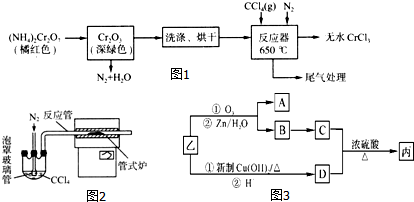
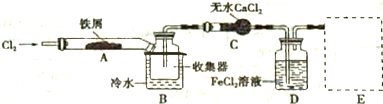
���� ��������ͼ֪���ظ���立ֽ������������������Cr2O3������ˮ����������ˮ��Ȼ��ϴ�ӡ���ɵõ�Cr2O3���ڼ��������£��ڷ�Ӧ���з�����ӦCr2O3+3CCl4�T2CrCl3+3COCl2�����Ȼ������������ڸ������ܱ�����������Ϊ��ֹ���Ȼ�����������ͨ�뵪�����ҳ���ĵ�����ʹ��Ӧ������ʽ¯�н��з�Ӧ�����õ���ˮ���Ȼ�����
��1���ظ���立ֽⲻ��ȫ�������ܺ����������������ʣ���Ϊ��NH4��2Cr2O7�Խۺ�ɫ�����Կ����ṩ��ɫ���жϣ�
��2����ΪCCl4�е�Ϊ76.8�棬�¶ȱȽϵͣ���˱�֤�ȶ���CCl4����������ͨ��ˮԡ������������������
��3�����������������Ȼ����ҳ��뵪����ʹ��Ӧ������ʽ¯�н��з�Ӧ��
��4��������Na2S2O3�ζ�����I2��I2����������ɫ���������һ��ʱ�������Һ��ɫ�ڰ�����ڲ���ɫ����ﵽ�ζ��յ㣻
���ζ�ʱ����֣��տ����ֲ���ɫ��ֹͣ�ζ������ʹ��Ʒ����ˮ���Ȼ��������������IJ������ƫ�ͣ�
����Һ�����ܽ��������������������I-������
��Cr2O72-��I-����������ԭ���ɵⵥ�ʺ����ӣ�
����CrԪ���غ㼰����ʽ�ɵù�ϵʽ2Cr3++��Cr2O72-��3I2��6Na2S2O3�����ݹ�ϵʽ���㣮
��� �⣺��1����Ϊ��NH4��2Cr2O7�Խۺ�ɫ�����һ��ϴ�ӵ�����Һ����ɫ��˵��ϴ�Ӹɾ���
�ʴ�Ϊ�����һ��ϴ�ӵ�����Һ����ɫ��
��2����ΪCCl4�е�Ϊ76.8�棬�¶ȱȽϵͣ���˱�֤�ȶ���CCl4����������ͨ��ˮԡ�����������������������¶ȼ�ָʾ�¶ȣ�
�ʴ�Ϊ��ˮԡ���ȣ����¶ȼ�ָʾ�¶ȣ���
��3�����������������Ȼ����ҳ��뵪����ʹ��Ӧ������ʽ¯�н��з�Ӧ��Ϊ��ֹ���Ȼ�����������ʹ��Ӧ������ʽ¯�н��з�Ӧ�����뵪����
�ʴ�Ϊ���Ͼ���Ӧװ���е�����������ʹ��Ӧ������ʽ¯�н��з�Ӧ��
��4��������Na2S2O3�ζ�����I2��I2����������ɫ�����Կ����õ�����ָʾ�����������һ��ʱ�������Һ��ɫ�ڰ�����ڲ���ɫ����ﵽ�ζ��յ㣻
���ζ�ʱ����֣��տ����ֲ���ɫ��ֹͣ�ζ������ʹ��Ʒ����ˮ���Ȼ�����Ӧ����ȫ�����Ե����������������IJ������ƫ�ͣ�
�ʴ�Ϊ�����ۣ����һ�ε���ʱ����ɫǡ����ȫ��ȥ���Ұ�����ڲ��ָ�ԭɫ��ƫ�ͣ�
����Һ�����ܽ��������������������I-������������ȥ�����ܽ������ʹ���ɵ�I2����������ƫ�ߵ����ʼ�����У�����Ҫԭ���ǣ���ȥ�����ܽ����������ֹ������I-����������ƫ�ߵ���
�ʴ�Ϊ����ȥ�����ܽ����������ֹ������I-����������ƫ�ߵ���
��Cr2O72-��I-����������ԭ���ɵⵥ�ʺ����ӣ����ӷ���ʽΪCr2O72-+6I-+14H+�T2Cr3++3I2+7H2O��
�ʴ�Ϊ��Cr2O72-+6I-+14H+�T2Cr3++3I2+7H2O��
����25.00mL��Һ��n��Cr3+������CrԪ���غ㼰����ʽ�ɵù�ϵʽ2Cr3++��Cr2O72-��3I2��6Na2S2O3�����ݹ�ϵʽ���㣮
2Cr3++��Cr2O72-��3I2��6Na2S2O3��
2 6
n��Cr3+�� 0.0250mol/L��0.021L
��n��Cr3+��=0.0250mol/L��0.021L��$\frac{1}{3}$������250mL��Һ��n�䣨Cr3+��=0.0250mol/L��0.021L��$\frac{1}{3}$��$\frac{250mL}{25mL}$=0.00175mol������CrԪ���غ��֪n��CrCl3��=n�䣨Cr3+��=0.00175mol��������Ʒ��m��CrCl3��=0.00175mol��158.5g/mol=0.2774g������Ʒ����ˮ���Ȼ�������������Ϊ$\frac{0.2774g}{0.3g}$��100%=92.5%��
�ʴ�Ϊ��92.5%��
���� ���⿼��ѧ���Ե�ʵ�鷽��ԭ�������������ۡ�������ɺ����IJⶨ���ζ�Ӧ�á���ѧ����ȣ���Ŀ�ѶȽϴ�����ʵ��ԭ���ǹؼ����ѵ��ǣ�4������㣬�״����ǻ���ʵ���������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | PbO2Ϊ��ԭ�������л�ԭ�� | |
| B�� | ���������뻹ԭ��������ʵ���֮��Ϊ5��2 | |
| C�� | ����1mol��Pb2+��ת�Ƶĵ��ӵ����ʵ���Ϊ2mol | |
| D�� | ���Ի�����MnO4-����ǿ�����ԣ���������ǿ��PbO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
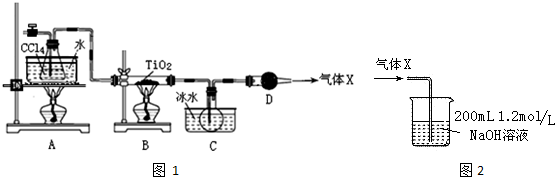
| ���� | �۵�/�� | �е�/�� | ���� |
| CCl4 | -23 | 76.8 | ��TiCl4���� |
| TiCl4 | -25 | 136 | ����ʪ������������ |
�鿴�𰸺ͽ���>>
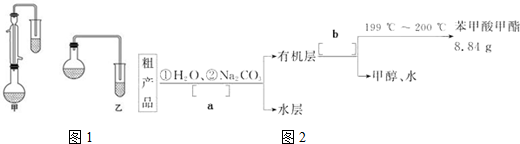
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶� | 25�� | 50�� | 95�� |
| �ܽ�� | 0.17g | 0.95g | 6.8g |
| ���� | �״� | ������ | ��������� |
| ��Է������� | 34 | 122 | 136 |
| �е�/�� | 64.7 | 249 | 199.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
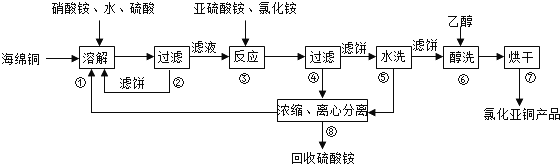
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
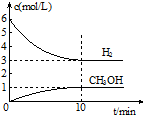 T��ʱ����1L���ܱ������г���2mol CO2��6mol H2��������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ•mol-1�����H2��CH3OH��g����Ũ����ʱ��仯�����ͼ��ʾ������˵������ȷ���ǣ�������
T��ʱ����1L���ܱ������г���2mol CO2��6mol H2��������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H=-49.0kJ•mol-1�����H2��CH3OH��g����Ũ����ʱ��仯�����ͼ��ʾ������˵������ȷ���ǣ�������| A�� | 0��10min��v��H2��=0.3 mol•L-1•min-1 | |
| B�� | T��ʱ��ƽ�ⳣ��K=$\frac{1}{27}$��CO2��H2��ת������� | |
| C�� | T��ʱ������32 g CH3OH����ʱ���ų�49.0 kJ������ | |
| D�� | �ﵽƽ��������¶Ȼ��ٳ���CO2���壬���������H2��ת���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com