
�˴Ź�����Ӱ��ǿ�����ڼ�����ϣ�������Ϊҩ���������ڼ��������ơ�Ϊ�������������������ҽѧ�����Ӧ���ṩ�˸��㷺��ǰ�����Ʊ����������������������£�
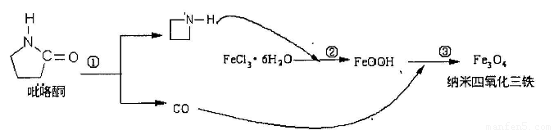
�����й��������������ǣ��� ��
A�������������������д�����Ϊҩ���������ڼ���������
B����Ӧ�۵Ļ�ѧ����ʽ�ǣ�6FeOOH+CO=2Fe3O4+3H2O+CO2
C������������������ɢ���ʵ��ܼ��У�������Һ��ɢ��ֱ���൱
D���ڷ�Ӧ�ڻ����������ÿ����Ǵٽ��Ȼ���ˮ��
 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��ش��������⣺
(1)����Ӧ�����ڼ��������½��еģ���A��(�ѧʽ)__________������Ӧ�����ڳ��������½��еģ���A��____________________(�ѧʽ)�������������������µõ�������C���ʣ���Ӧ��ת�Ƶĵ�����֮��Ϊ____________________.
(2)H��;�㷺������������ӡˢ��·��ʴ��������ֹѪ������ˮ��.����Cu����C���õ������Ӳ�����H��Һ�У����γ�ԭ��أ����������缫��Ӧ����ʽΪ�� ____________________.�����ӷ���ʽΪ��____________________.F��B��Ӧ�����ӷ���ʽΪ�� ____________________.
(3)���ⶨAΪ��Ԫ���ᣬ�����Ա�̼������д��A��ˮ��Һ�еĵ��뷽��ʽ�� ____________________.A�백����Ӧ���ɵ���ʽ�εĻ�ѧʽΪ��____________________.��������MnO2�����ữ���A��Һ�У�MnO2�ܽ����Mn2+���÷�Ӧ�����ӷ���ʽΪ�� ____________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08��������ʮ��У�¿�)��ͼ���漰�����ʾ�Ϊ��ѧ��ѧ�г��������ʣ�����C��D��EΪ���ʣ�����Ϊ�������������F������Ͽ���Ϊ�˴Ź�����Ӱ��ǿ���������ڼ�������Ϻ�ҩ�����壬����֮���������ת����ϵ�����ֲ�����ʡ�ԣ���

��ش��������⣺
��1������Ӧ�����ڼ��������½��еģ���A�ǣ��ѧʽ��____________������Ӧ�����ڳ��������½��еģ���A�ǣ��ѧʽ��_____________�������������������µõ�������C���ʣ���Ӧ��ת�Ƶĵ�����֮��Ϊ_____________��
��2��H��;�㷺������������ӡˢ��·��ʴ��������ֹѪ������ˮ�ȡ�����Cu����C���õ������Ӳ�����H��Һ�У����γ�ԭ��أ����������缫��Ӧ����ʽΪ��
___________________ ______�������ӷ���ʽΪ��__________ _______________��
F��B��Ӧ�����ӷ���ʽΪ��________ ��
��3���羭�ⶨAΪ��Ԫ���ᣬ�����Ա�̼������д��A��ˮ��Һ�еĵ��뷽��ʽ��______________________________________________________________________________��
A�백����Ӧ���ɵ���ʽ�εĻ�ѧʽΪ��_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08�㽭����ʮ��У�¿�)��ͼ���漰�����ʾ�Ϊ��ѧ��ѧ�г��������ʣ�����C��D��EΪ���ʣ�����Ϊ�������������F������Ͽ���Ϊ�˴Ź�����Ӱ��ǿ���������ڼ�������Ϻ�ҩ�����壬����֮���������ת����ϵ�����ֲ�����ʡ�ԣ�

��ش��������⣺
��1������Ӧ�����ڼ��������½��еģ���A�ǣ��ѧʽ��____________������Ӧ�����ڳ��������½��еģ���A�ǣ��ѧʽ��_____________�������������������µõ�������C���ʣ���Ӧ��ת�Ƶĵ�����֮��Ϊ_____________��
��2��H��;�㷺������������ӡˢ��·��ʴ��������ֹѪ������ˮ�ȡ�����Cu����C���õ������Ӳ�����H��Һ�У����γ�ԭ��أ����������缫��Ӧ����ʽΪ��_________�������ӷ���ʽΪ��_______��
F��B��Ӧ�����ӷ���ʽΪ��___________��
��3���羭�ⶨAΪ��Ԫ���ᣬ�����Ա�̼������д��A��ˮ��Һ�еĵ��뷽��ʽ��___��
A�백����Ӧ���ɵ���ʽ�εĻ�ѧʽΪ��_________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com