
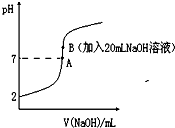
 ����ᣨHF����һԪ���ᣬ��ˮ��Һ�еĵ��뷽��ʽΪ��HF?H++F-��25���£���20mL0.2mol?L-1��������еμ�0.2mol?L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ��
����ᣨHF����һԪ���ᣬ��ˮ��Һ�еĵ��뷽��ʽΪ��HF?H++F-��25���£���20mL0.2mol?L-1��������еμ�0.2mol?L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ��| n(�ѵ�����������) |
| n(ȫ�����������) |
| c(H+)?c(F-) |
| c(HF) |
| 0.01mol/L |
| 0.2mol/L |
| c(F-)?c(H+) |
| c(HF) |
| 0.01��0.01 |
| 0.2-0.01 |
| c(F-)?c(H+) |
| c(HF) |


 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
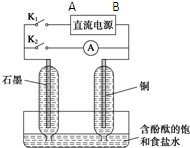 ij��ȤС�������ͼ��ʵ��װ�ã�ʵ��ʱ���ȶϿ�K2���պ�K1�������������ݲ�����һ��ʱ��Ͽ�K1���պ�K2�����ֵ�����Aָ��ƫת��
ij��ȤС�������ͼ��ʵ��װ�ã�ʵ��ʱ���ȶϿ�K2���պ�K1�������������ݲ�����һ��ʱ��Ͽ�K1���պ�K2�����ֵ�����Aָ��ƫת���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ں͢� | B���٢ڢ� |
| C���ڢܢݢ� | D���ڢۢݢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1mol/L |
| B��2mol/L |
| C��2.5mol/L |
| D��3mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ʯ��ʵ�� |
| B����ȩ��֬���Ʊ� |
| C���Ҵ���Ũ���������·�����ȥ��Ӧ |
| D�����������Ʊ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��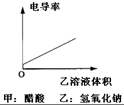 |
B�� |
C��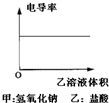 |
D��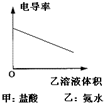 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com