
| Ԫ �� | M | F | |
| ���� ��kJ•mol-1�� | I1 | 717 | 759 |
| I2 | 1509 | 1561 | |
| I3 | 3248 | 2957 | |
| �۵�/K | �е�/K | ��״��ʱ��ˮ�е��ܽ�� | |
| H2S | 187 | 202 | 2.6 |
| H2C2 | 272 | 423 | ������Ȼ��� |
���� A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵ�ԭ������������������A��Cԭ�ӵ�L����2��δ�ɶԵ�����Aԭ������С��C����A��C��C��OԪ�أ�Bԭ����������A��С��C����B��NԪ�أ�
D��Eͬ���壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ����DΪMgԪ�أ�
F3+����M��3d�������Ϊ����״̬����Fԭ�Ӻ��������=2+8+8+5+3=26������FΪFeԪ�أ�
��1��FΪFeԪ�أ���ԭ�Ӻ�����26�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽ��FeԪ��λ�ڵ������ڵ�VIII�壻
��2��ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA���һ�����ܴ���������Ԫ�أ�
��3��ԭ�ӹ���е��Ӵ���ȫ����ȫ�ա�����ʱ���ȶ���
��4��K�������������ܶѻ���K��Fe�Ķѻ�ģ����ͬ������FeΪ�����������ܶѻ���Feԭ����λ����8���þ�������ԭ�Ӹ���=8��$\frac{1}{8}$+1=2��
��5��������������ʵ��ܽ��ԣ�
��� �⣺A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵ�ԭ������������������A��Cԭ�ӵ�L����2��δ�ɶԵ�����Aԭ������С��C����A��C��C��OԪ�أ�Bԭ����������A��С��C����B��NԪ�أ�
D��Eͬ���壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ����DΪMgԪ�أ�
F3+����M��3d�������Ϊ����״̬����Fԭ�Ӻ��������=2+8+8+5+3=26������FΪFeԪ�أ�
��1��FΪFeԪ�أ���ԭ�Ӻ�����26�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d64s2��FeԪ��λ�ڵ������ڵ�VIII�壬
�ʴ�Ϊ��1s22s22p63s23p63d64s2��VIII��
��2��ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA���һ�����ܴ���������Ԫ�أ�C��N��OԪ��λ��ͬһ����Ԫ�أ�Cλ�ڵ�IVA�塢Nλ�ڵ�VA�塢Oλ�ڵ�VIA�壬���Ե�һ������˳����C��O��N���ʴ�Ϊ��C��O��N��
��3��ԭ�ӹ���е��Ӵ���ȫ����ȫ�ա�����ʱ���ȶ���Mn2+��3d��������Ų�Ϊ����״̬���ȶ���
�ʴ�Ϊ��Mn2+��3d��������Ų�Ϊ����״̬���ȶ���
��4��K�������������ܶѻ���K��Fe�Ķѻ�ģ����ͬ������FeΪ�����������ܶѻ���Feԭ����λ����8���þ�������ԭ�Ӹ���=8��$\frac{1}{8}$+1=2���ʴ�Ϊ��8��2��
��5��������������ʵ��ܽ��ԣ�H2O2���Ӽ�����������ˮ���ӿ��γ���������������֮�䲻���γ��������ˮ����Ҳ�����γ�����������ܽ��Խ�С
�ʴ�Ϊ��H2O2���Ӽ�����������ˮ���ӿ��γ������
���� ���⿼��λ�ýṹ�������ϵ��Ӧ�ã��漰�������㡢�������һ�����ܹ��ɡ�ԭ�Ӻ�������Ų���֪ʶ�㣬Ϊ��Ƶ���㣬ע�⣨5�������ֻӰ���������ʣ���Ӱ�컯ѧ���ʣ�


 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | ʵ�鷽�� | ʵ������ |
| �� | ��������ˮ����ʢ������NaBr��Һ���Թ��У����ٵ����������Ȼ�̼���� | ������ˮ����Һ����ɫ��Ϊ��ɫ���������Ȼ�̼��ˮ����ɫ��dz�����Ȼ�̼�㣨�²㣩��Ϊ�Ⱥ�ɫ |
| �� | ��������ˮ����ʢ������KI��Һ���Թ��У� ���ٵ����������Ȼ�̼���� | ������ˮ����Һ����ɫ��Ϊ��ɫ���������Ȼ�̼��ˮ����ɫ��dz�����Ȼ�̼�㣨�²㣩��Ϊ�Ϻ�ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ױ������ϵ�һ����ԭ�ӱ�-C3H7ȡ�������ò�����6�� | |
| B�� | ��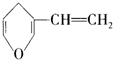 ��Ϊͬ���칹��ķ����廯������4�� ��Ϊͬ���칹��ķ����廯������4�� | |
| C�� | ��֪�����������֣�������д�������ɶ϶�����ʽΪC5H10O��ȩӦ��4�� | |
| D�� | �ƵĽṹ��ʽΪ 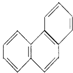 ��һ���������������ᷴӦ����������5��һ����ȡ���� ��һ���������������ᷴӦ����������5��һ����ȡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
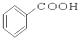
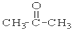 ��CH3CH2Br ��
��CH3CH2Br ��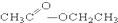 ��
��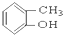 ��
��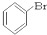 ��
��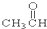 ��
��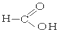 ��
��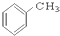 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2014�����ǹ��ұ���������������˫��ˮ�����������Һ������ȫ���ò�����Ⱦ�ԣ�������ԭ����Ư����������ˮ��ͬ | |
| B�� | �������������Һ�л�ȡ��������茶��壬�����������ᾧ��Ҳ��������ȴ�ᾧ | |
| C�� | iPhone6�����ɫ����Ϊ���DZ�������������������Ϊ�����Ľ��������Ȳ��� | |
| D�� | �Ƚ�ȥ���������ȥ�������ϸͭ˿��������ͬŨ�ȵ����ᷴӦ���ʿ���ʱ�����Լ�K3[Fe��CN��6]��Һ���۲�������Χ������ɫ�����Ŀ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������м���Ũ�������ַ�������˵��Ũ���������ˮ�� | |
| B�� | ��ij��Һ�м����Ȼ�����Һ��ϡ���ᣬ���ɰ�ɫ��������ԭ��Һһ������SO42- | |
| C�� | �����£���ͭ����Ũ�����������Ա仯��˵��ͭ�����Ũ�����жۻ� | |
| D�� | Ũ�����ڹ����±�ƣ�֤������ȶ����Ҳ����к���ɫ���������Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C60����ʯһ������ԭ�Ӿ��� | |
| B�� | �ɱ������ƻ��˹��ۼ� | |
| C�� | ���ۻ�������һ���������Ӽ� | |
| D�� | �Ȼ�������ˮ�ܵ����H+��Cl-�������Ȼ��������ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���õ����еĽ������� | B�� | ��ֹ���������Ⱦ | ||
| C�� | �ϵ�������������������ʹ�� | D�� | �������зǽ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | VOSO4 | V2O5 | NH4VO3 | ��VO2��2SO4 |
| �ܽ��� | ���� | ���� | ���� | ���� |
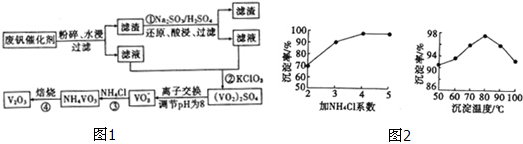
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com