
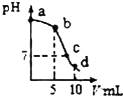
 25��ʱ����10mL0.2mol•L-1NaCN��Һ�м���0.2mol•L-1���ᣬ��ҺpH��������������仯�������ͼ��ʾ����������˵������ȷ���ǣ���������֪��Ka��HCN��=6.4��10-10��
25��ʱ����10mL0.2mol•L-1NaCN��Һ�м���0.2mol•L-1���ᣬ��ҺpH��������������仯�������ͼ��ʾ����������˵������ȷ���ǣ���������֪��Ka��HCN��=6.4��10-10��| A�� | a��ʱ��CN-����Ũ�ȴ��������� | B�� | b��ʱ��c��HCN����c��CN-�� | ||
| C�� | c��ʱ��c��Na+��=c��Cl-��+c��CN-�� | D�� | d��ʱ����Һ��c��H+����8��10-5mol•L-1 |
���� A��a��ΪNaCN��Һ����CN-���ӵ�ˮ��̶Ƚ�С������Ũ�Ƚӽ�0.2mol/L����������Ũ�ȶ�С��a��
B��b��Ϊ��Ũ�ȵ�NaCN��HCN����Һ�ʼ��ԣ���HCN�ĵ���̶�С��NaCN��ˮ��̶ȣ�
C��c����ҺΪ���ԣ���c��H+��=c��OH-�������ݵ���غ��жϣ�
D��d��Ϊ0.1mol/L��HCN��Һ�����������Ũ�ȣ�Ȼ����HCN�ĵ���ƽ�ⳣ�����м��㣮
��� �⣺A��a��ΪNaCN��Һ��HCNΪ���ᣬCN-���ӵ�ˮ��̶Ƚ�С����CN-��Ũ�Ƚӽ�0.2mol/L������������������ļ��룬CN-���ӵ�Ũ��Ѹ�ټ��٣�����a��CN-����Ũ�ȴ��������㣬��A��ȷ��
B������ͼ���֪��b�����5mL���ᣬ��Ӧ������Ϊ��Ũ�ȵ�NaCN��HCN�����Һ��pH����7���ʼ��ԣ���HCN�ĵ���̶�С��NaCN��ˮ��̶ȣ�����c��HCN����c��CN-������B��ȷ��
C��c����Һ��pH=7�������ԣ���c��H+��=c��OH-�������ݵ���غ��֪��c��Na+��=c��Cl-��+c��CN-������C��ȷ��
D��d��ʱ����10mLHCl���ᣬ��Ӧ����Һ������Ϊ0.1mol/L��NaCl��0.1mol/L��HCN������Һ��������Ũ��Ϊx������HCN?CN-+H+��֪��Һ��c��CN-����c��H+��=x��c��HCN��=0.1-x��x����Ka��HCN��=$\frac{x��x}{0.1}$=6.4��10-10�����x=8��10-6mol•L-1����D����
��ѡD��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷͼ������������Ϊ���ؼ���ע��������Һ���������ҺpH�Ĺ�ϵ���ܹ����õ���غ㡢�����غ㼰�ε�ˮ��ԭ���жϸ�����Ũ�ȴ�С������������ѧ���ķ������������Ӧ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯī��Χ�д�����Na+ | |
| B�� | ������������ | |
| C�� | ����ͨ���������Һ��ʯī���������� | |
| D�� | �����缫����������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ȼ��ʱ������ȫ��ͬ | |
| B�� | ��ȼǰ������Ҫ�鴿 | |
| C�� | ����ȼ�ջ���ʵ���ɫ����ϩȼ�ջ�������� | |
| D�� | ����ȼ��ʱ���к������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Na[Al��OH��4]��Һ��ͨ�����CO22[Al��OH��4]-+CO2=2Al��OH��3��+CO32-+H2O | |
| B�� | Na2SiO3��Һ��ϡ������SiO32-+2H+=H2SiO3�� | |
| C�� | ����CuSO4��Һ��Ӧ2Na+Cu2+=Cu+2Na+ | |
| D�� | ��Na2SO3��Һ�еμ�˫��ˮ H2O2+SO32-=SO42-+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
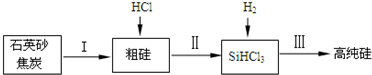
| ���� | Si | SiCl4 | SiHCl3 | SiH2Cl2 | SiH3Cl | HCl | SiH4 |
| �е�/�� | 2355 | 57.6 | 31.8 | 8.2 | -30.4 | -84.9 | -111.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ѡ�õ����� | |||||
| ���ӵ�ҩƷ�����Ҫ�IJ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʹ��Һ��ʯ����������ȫ�ž�������Ⱦ | |
| B�� | Ϊ��ֹ���д�Ⱦ���ɽ������Ŵ��رպ���ʳ��Ѭ������������ | |
| C�� | �Ȼ����Ǽ�ͥ���õķ�����������������ʳƷ | |
| D�� | ҽ���ϳ����������Ϊ70%��75%�ľƾ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2.4g����þ���þ����ʱʧȥ�ĵ�����Ϊ0.1NA | |
| B�� | ��״���£�11.2L CCl4�����ķ�����Ϊ0.5NA | |
| C�� | 0.5mol/L AlCl3��Һ��Cl-����ĿΪ1.5NA | |
| D�� | 17g�����к��еĵ�����Ϊ10NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com