
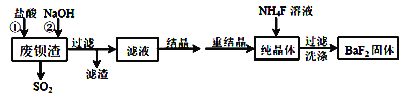
����Ŀ�����÷ϱ�������Ҫ�ɷ�ΪBaS2O3��������SiO2��Ϊԭ�������ߴ����������������£�
��֪��Ksp(BaS2O3)=6.96��10-11��Ksp(BaF2)=1.0��10-6
��1����������ʱ������SO2�⣬���е���ɫ�������ɡ��÷�Ӧ�����ӷ���ʽΪ___________________________________________________________��
��2����Һ����Ҫ�ɷ���____________�����ѧʽ��
��3����ҵ�Ͽ��ð�ˮ����SO2����ͨ�����ʹ��ת��Ϊ�̬���ʡ���ת�����������뻹ԭ�������ʵ���֮��Ϊ_____________��
��4������NaOH��Һ��Ŀ�����к��������ᣬ�����˹�������ԭ����____________�������ӷ�Ӧ����ʽ��ʾ����
��5������BaF2�ķ�Ӧ�Ļ�ѧ����ʽΪ_________________________��
�����÷�Ӧ�¶ȹ��ߣ��������c(F-)���͵�ԭ����_______________________��
���о��������ʵ�����NH4F�ı������������BaF2�IJ��ʺʹ��ȡ���Ũ��Ϊ0.1 molL-1��BaCl2��Һ��0.22molL-1NH4F��Һ�������ϣ�������Һ��c(Ba2+)=__________��
���𰸡�BaS2O3+2H+��Ba2++S��+SO2��+H2O BaCl2��NaCl 1�U2 2OH-+SiO2��SiO32-+H2O BaCl2+2NH4F��BaF2��+2NH4Cl �¶Ƚϸߴٽ�F-ˮ�⣬ʹc(F-)���� 0.01 molL-1
��������
��������ͼ�еIJ���ͷ�Ӧ������漰�ķ�Ӧ�����ݵ�ʧ����������ȷ����������뻹ԭ�������ʵ���֮�ȣ������ܶȻ�������Һ�е�����Ũ�ȡ�
(1)��������ʱ������SO2�⣬���е���ɫ��������,��÷�Ӧ�����ӷ���ʽΪ��BaS2O3+2H+��Ba2++S��+SO2��+H2O��
��2����Һ����Ҫ�ɷ���������BaS2O3��Ӧ���õĿ����Բ��BaCl2��NaCl��
��3����ҵ�Ͽ��ð�ˮ����SO2����ͨ�����ʹ��ת��Ϊ�̬���ʣ���Ӧ�л�ԭ��Ϊ��������������Ϊ������SԪ�ػ��ϼ�����2�������ϼ۽���2���ʸ�ת�����������뻹ԭ�������ʵ���֮��Ϊ��1:2��
��4���������������������������Ӧ�����ӷ���ʽΪ��2OH-+SiO2��SiO32-+H2O ����5����Һ�к����Ȼ�������NH4F��Ӧ����BaF2�Ļ�ѧ����ʽΪ��BaCl2+2NH4F��BaF2��+2NH4Cl ��
�����÷�Ӧ�¶ȹ��ߣ��������c(F-)���͵�ԭ���ǣ��¶Ƚϸߴٽ�F-ˮ�⣬ʹc(F-)���ͣ�
��Ksp(BaF2)= c2(F-)��c(Ba2+)=1.0��10-6��c(F-)=c(NH4F)/2=0.22molL-1/2=0.11 molL-1���� c(Ba2+)= 0.01 molL-1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ӿ���Ч��ͻ����������ǣ�������ȡCu��NO3��2���˲��õķ�����
A. Cu![]() Cu��NO3��2
Cu��NO3��2
B. Cu![]() Cu��NO3��2
Cu��NO3��2
C. Cu![]() CuO
CuO![]() Cu��NO3��2
Cu��NO3��2
D. Cu![]() CuCl2
CuCl2![]() Cu��NO3��2
Cu��NO3��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ4A(s)+3B(g)==2C(g)+D(g)����2min��B��Ũ�ȼ���0.6mol��L-1������˵����ȷ����
A. ��A��ʾ�ķ�Ӧ������0.4mol��L-1��min-1
B. ��2minĩ�ķ�Ӧ��������B��ʾ��0.3 mol��L-1��min-1
C. �ֱ���B��C��D��ʾ��Ӧ�����ʣ����ֵ��3:2:1
D. ����2min��B��C������Ũ������С��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ý�屨��һ��������ˮ����װ����������װ�ÿ�����һ�����ォ�л���ˮ�Ļ�ѧ��ֱ��ת��Ϊ���ܣ���װ�õĹ�����ͼ��ʾ������˵������ȷ����(����)
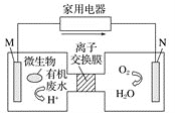
A. װ������·�м�ͷ�ķ�����������ķ���
B. ��װ��Ϊԭ���װ�ã�����NΪ����
C. ��װ��Ϊ����װ�ã�����MΪ����
D. ���л���ˮ�к��������ǣ���M�缫�����ĵ缫��ӦʽΪC6H12O6��6H2O��24e��![]() 6CO2��24H��
6CO2��24H��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���a mol N2��b mol H2�Ļ������ͨ��һ���̶��ݻ����ܱ������У��������·�Ӧ��N2 (g) �� 3 H2(g)![]() 2NH3(g)
2NH3(g)
��1������Ӧijʱ��tʱ��nt (N2) =" 13" mol��nt(NH3) =" 6" mol����a =__________mol��
��2����Ӧ��ƽ��ʱ�������������Ϊ716.8 L������£�������NH3�ĺ���(�������)Ϊ25%��ƽ��ʱNH3�����ʵ���__________��
��3��ԭ���������ƽ��������������ʵ���֮�ȣ�д����������ȡ���ͬ����n(ʼ)��n(ƽ) =__________��
��4��ԭ��������У�a��b =__________��
��5���ﵽƽ��ʱ��N2��H2��ת����֮�ȣ���(N2)�æ�(H2)= __________��
��6��ƽ���������У�n(N2)��n(H2)��n(NH3) =__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ԭ���������������ֶ�����Ԫ��(����ĸx�ȱ�ʾ)ԭ�Ӱ뾶����Դ�С��������ۻ�����۵ı仯����ͼ��ʾ��

�����жϳ���Ԫ�ػش����⣺
(1)f�����ڱ��е�λ����____________��
(2)�Ƚ�d��e�������ӵİ뾶��С(�û�ѧʽ��ʾ����ͬ)��________>________���Ƚ�g��h������������Ӧˮ���������ǿ����____________>____________��
(3)��ѡ����Ԫ�����һ����ԭ�ӹ��ۻ����д�������ʽ��________________________��
(4)д��e�ĵ����ڿ�����ȼ�����ò���ĵ���ʽ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������֮������ϵ�������(����)
A. CH3CH2CH2CH2CH3�� ��Ϊͬ���칹��
��Ϊͬ���칹��
B. �ɱ��ͱ�Ϊͬһ������
C. CH3CH3��CH3CH2CH3��Ϊͬϵ��
D. 12C��14C��Ϊͬλ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��ZΪ������Ԫ�أ�Xԭ�ӵ�����������Z��Y��������֮�ͣ�Z��Yλ��ͬһ���ڣ�Yԭ�Ӻ�����3��δ�ɶԵ��ӣ��ǽ���Z��һ�ֹ��嵥�ʿɵ��硣�ش��������⣺
��1��Y�����ڱ��е�λ���ǵ�______���ڵ�_____�壬��ԭ�ӽṹʾ��ͼΪ_______________��Y��Z֮���γɵĻ�ѧ������__________��
��2��X��Y��Z����Ԫ����ԭ�Ӱ뾶������__________(��Ԫ�ط���)��X���ʼȿ������ᷴӦ���ֿ���������������Һ������������Ϊ__________(�����ʽ)����������Y���ʷ�Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��3��Z�����������ĵ���ʽΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��CH3��CH===CH2��HBr�D��![]() (��Ҫ����),
(��Ҫ����),
1 molij��A���ȼ�պ���Եõ�8 mol CO2��4 mol H2O������A�ڲ�ͬ�������ܷ�����������ʾ��һϵ�б仯��

(1)A�Ļ�ѧʽ��______��A�Ľṹ��ʽ��__________��
(2)������Ӧ�У�����____��Ӧ������____��Ӧ��(�Ӧ����)
(3)д��C��D��E��H���ʵĽṹ��ʽ��
C__________��D__________��
E__________��H__________��
��4��д��D��F��Ӧ�Ļ�ѧ����ʽ__________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com