
��������Ӱ�����ǵ������뽡����ij�����������п��ܺ������¿����������ӣ�Na����NH4+��Mg2����Al3����SO42����NO3-��Cl����ijͬѧ�ռ��˸õ���������������Ҫ��Ԥ�������������Һ����Ʋ����������ʵ�飺

��֪��3NO3-��8Al��5OH����2H2O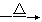 3NH3����8AlO2-
3NH3����8AlO2-
�������ϵ�ʵ�����������ͬѧ�ó��Ľ��۲���ȷ����( )
A�������п϶�����NH4+��Mg2����SO42����NO3-
B��������һ������Al3��
C�������п��ܴ���Na����Cl��
D���������п��ܴ���NaNO3��NH4Cl��MgSO4

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ������һ������Կ��Ի�ѧ�Ծ��������棩 ���ͣ��ƶ���
X��Y��Z��R��Q��M�����ֶ�����Ԫ�أ�ԭ��������������X��ԭ�Ӱ뾶��С��Ԫ�أ�Y����̬�⻯����ʹʪ��ĺ�ɫʯ����ֽ������ZΪ�ؿ��к�������Ԫ�أ�R��Xͬ���壻Y��R��Q����������֮��Ϊ8��M�ĵ��ʻ���ɫ�к����塣��ش��������⣺
(1)R��Ԫ�����ڱ��е�λ��Ϊ___________��
(2)Z��Q��M�����Ӱ뾶�ɴ�С��˳��Ϊ(дԪ�����ӷ���)___________��
(3)X��Y��Z����Ԫ���γ���������ˮ��Һ�����Ե�ԭ��(�����ӷ���ʽ��ʾ)________________��
��Һ����������Ũ���ɴ�С��˳��Ϊ________________��
(4)YX4M�ĵ���ʽΪ___________��Q3Y2��ˮ�ɾ��ҷ�Ӧ���������������壬��Ӧ�Ļ�ѧ����ʽΪ
____________________��
(5)X��Z��Ԫ���γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ������к��еĻ�ѧ������Ϊ_______________________��
(6)M�ĵ�����R������������Ӧ��ˮ���ﷴӦ�����ӷ���ʽΪ_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ɹŸ����ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
NaCNΪ�綾���ij��ȤС������ϵ�֪��ʵ�������NaCN��Һ����Na2S2O3��Һ���нⶾ���٣����ǿ�չ����������ʵ�飬�����Ҫ��ش����⣺
ʵ�����������ƾ��壨Na2S2O3��5H2O�����Ʊ���
��֪Na2S2O3��5H2O���Ȳ��ȶ�������48�漴��ʼ��ʧ�ᾧˮ������Na2CO3��Na2S���ʵ���֮��Ϊ2��1�Ļ����Һ��SO2����Ϊԭ�ϣ�������ͼװ���Ʊ�Na2S2O3��5H2O��
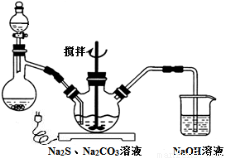
��1����Na2S��Na2CO3����ӦҪ��ı���һ������������ƿ�У�ע��150 mL����ˮʹ���ܽ⣬��������ƿ�м���Na2SO3���壬�ڷ�Һ©����ע��____________��������ѡ�������ĸ����������ͼ��װ��װ�ã����з�Ӧ��
A��ϡ���� B��Ũ���� C��70%������ D��ϡ����
��2��pHС��7������Na2S2O3��Һ�ı��ʷ�Ӧ������ֵ���ɫ���ǡ���ӦԼ��Сʱ������ҺpH�ӽ���С��7ʱ������ֹͣͨ���ͼ��ȡ����ͨ��SO2�����������Ļ�ѧ��Ӧ����ʽΪ________________��
ʵ���Ʒ���ȵļ�⣺
��3����֪��Na2S2O3��5H2O��Ħ������Ϊ248 g/mol��2Na2S2O3+I2=2NaI+Na2S4O6��ȡ������Ʒa g����ˮ�ܽ���뼸�ε�����Һ����0.010 mol/L��ˮ�ζ����յ�ʱ�����ĵ�ˮ��Һv mL���ٵζ��յ�������� ���ڸ���Ʒ������______________________��
��4���ζ������п������ʵ����ƫ�͵���___________________��
A����ƿδ��Na2S2O3��Һ��ϴ
B����ƿ����Һ����������ֹͣ�ζ������ж���
C���ζ��յ�ʱ���Ӷ���
D���ζ��ܼ����ڵζ�ǰ�����ݣ��ζ��յ㷢������
ʵ����ж���ˮ�Ĵ�����
��5����ȤС���ͬѧ�ڲ�ȡϵ�з�����ʩ����ʦ��ָ���½�������ʵ�飺
��װ��2 mL 0.1 mol/L ��NaCN��Һ���Թ��еμ�2 mL 0.1mol/L ��Na2S2O3��Һ������Ӧ��ǡ����ȫ��Ӧ��������������ȡ��Ӧ�����Һ��������ʢ��10 mL 0.1 mol/L FeCl3��Һ��С�ձ�����Һ����Ѫ��ɫ����д��Na2S2O3�ⶾ�����ӷ�Ӧ����ʽ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ɹŸ����ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪�����£�Ksp(AgCl)��1.8��10��10��Ksp(Ag2CrO4)��1.9��10��12 ������������ȷ����
A��AgCl�ڱ���NaCl��Һ�е�Ksp���ڴ�ˮ�е�С
B����AgCl������Һ�м���NaBr��Һ����ɫ����ת��Ϊ����ɫ��˵��Ksp(AgCl)<Ksp(AgBr)
C����0.001 mol��L��1AgNO3��Һ�ֱ����0.001 mol��L��1��KCl��0.001 mol��L��1��K2CrO4��Һ���Ȳ���Ag2CrO4����
D����AgCl������Һ�еμ�Ũ��ˮ�������ܽ⣬˵��AgCl���ܽ�ƽ�������ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ɹŸ����ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ��ƶ���
�л���F(C11H12O2)���ڷ����������ʣ���������·�ߺϳɣ����� ����C �Ǽױ�������B��һ�������¿��Է����Ӿ۷�Ӧ���γɸ߷��ӻ����

��ش�
��1��A�й����ŵ�������_____________________
��2��C���ʵĽṹ��ʽ��______________________����Ӧ�۵�������_____________________��
��3��д��B��һ�������·����Ӿ۷�Ӧ�γɸ߷��ӻ�����Ļ�ѧ����ʽ��_____________________��
��4����Ӧ�Ļ�ѧ����ʽΪ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ɹŸ����ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��ֻ�ص���������������ĸ���Ӧ�������ж�����4�����ʵ���������ǿ��������ȷ˳���ǣ� ��
��Cl2+2KI=2KCl+I2
��2FeCl2+Cl2=2FeCl3
��2FeCl3+2HI=2FeCl2+2HCl+I2
��H2S+I2=S+2HI
A��H2S>I2>Fe3+>Cl2 B��Cl2> Fe3+ > I2 > H2S
C��Fe3+ > Cl2 > H2S > I2 D��Cl2>I2>Fe3+> H2S
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ɹŸ����ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������л���Ľṹ�������йص���������ȷ����( )
A���������ϩ��������������Ӧ,��Ӧ���Ͳ�ͬ
B�����ǡ���֬�������ʶ�����ˮ��
C����ϩ������ϩ������ͨ���ۺϷ�Ӧ�õ��߷��Ӳ���
D���Ҵ��������ж�����̼��˫��,���߿��Է���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������9���ۺ��������Ի�ѧ�Ծ��������棩 ���ͣ��ƶ���
��ͼ������A��M��һ�������µ�ת����ϵ�����ֲ��P��Ӧ����δ�г��������У�I���ɵ�������Ԫ����ɵĵ������۵���ߵĽ�����K��һ�ֺ���ɫ���壮

����д���пհף�
��1�������ڱ��У���ɵ���G��Ԫ��λ�ڵ� ���� �壮
��2���ڷ�Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ ��
��3���ڷ�Ӧ�ڡ��ۡ��ޡ����У������ڻ��Ϸ�Ӧ�����ڷ�������ԭ��Ӧ���� ������д��ţ�
��4����Ӧ�ܵ����ӷ���ʽ�ǣ� ��
��5����������D��KNO3��KOH���ڣ����Ƶ�һ�֡���ɫ��������Ч��ˮ��K2FeO4��������أ���ͬʱ������KNO2��H2O���÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��������ʵ���Ũ��Ϊc mol/L,���ʵ���������Ϊa%�������Һ���ܶȣ�g/cm3��Ϊ( )
A��63c/a B��6.3a/c C��6.3c/a D��63a/c
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com