
����A����Ũ��ˮ�ֱ��Ca(NO3)2��(NH4)2HPO4��Һ��pHԼΪ12���ھ��ҽ����£���(NH4)2HPO4��Һ��������Ca(NO3)2��Һ�С�
����B�����ҽ����£���H3PO4��Һ�����μӵ�Ca(OH)2����Һ�С�
3�ָ��ε��ܽ������ҺpH�ı仯����ͼ��ʾ��ͼ���������Ǹ�����Ũ�ȵĶ��������ش��������⣺

��1����ɷ���A�ͷ���B���Ʊ�Ca5(PO4)3OH�Ļ�ѧ��Ӧ����ʽ��
��5Ca(NO3)2+3(NH4)2HPO4+4NH3��H2O=Ca5(PO4)3OH��+ +
��5Ca(OH)2+3H3PO4=
��2���뷽��A��ȣ�����B���ŵ��� ��
��3������B�У����H3PO4��Һ�μӹ��죬�ƵõIJ��ﲻ������ԭ����
��
��4��ͼ����ʾ3�ָ��������������ȶ��Ĵ�����ʽ�� ���ѧʽ����
��5����մ���������ϣ���ø�������²����������ʣ������ȣ�ݡ���ϻ�ѧƽ���ƶ�ԭ����������ԭ�� ��
��1����10NH4NO3 3H2O ��Ca5(PO4)3OH��+9H2O
��2��Ψһ������Ϊˮ�����ռ�
��3����ӦҺ�ֲ����Թ�����CaHPO4����
��4��Ca5��PO4��3OH
��5����������ʹ�����ܽ�ƽ�⣺Ca5(PO4)3OH(s)![]() 5Ca2+(aq)+3
5Ca2+(aq)+3![]() (aq)+OH��(aq)�����ƶ�������Ca5��PO4��3OH�ܽ⣬���ȣ��
(aq)+OH��(aq)�����ƶ�������Ca5��PO4��3OH�ܽ⣬���ȣ��
������������һ����ѧƽ�������ܵ���ʵ��ܽ�ƽ����ۺ��⡣�ݷ�Ӧǰ�������غ���ƽδ֪����
5Ca(NO3)2+3(NH4)2HPO4+4NH3��H2O===Ca5(PO4)3OH��+10NH4NO3+3H2O��
Ca(OH)2+3H3PO4===Ca5(PO4)3OH��+9H2O������A�뷽��B�Ƚϣ�����B���ŵ��ǹ��ռ�ֻ��һ�ָ�����H2O������Ӧ�μ�H3PO4���죬Ca5(PO4)3OH��H3PO4��Ӧ����CaHPO4��Ca5(PO4)3OH����Һ�д������г����ܽ�ƽ�⣺
Ca5(PO4)3OH(s)![]() 5Ca2+(aq)+3PO
5Ca2+(aq)+3PO![]() (aq)+OH��(aq)��������Һ��ƽ�������ƶ���ʹCa5(PO4)3OH�ܽ⣬���ȣ�ݡ�
(aq)+OH��(aq)��������Һ��ƽ�������ƶ���ʹCa5(PO4)3OH�ܽ⣬���ȣ�ݡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 5Ca2+��aq��+3PO43-��aq��+OH-��aq�������ƶ�������Ca5��PO4��3OH�ܽ⣬���ȣ��
5Ca2+��aq��+3PO43-��aq��+OH-��aq�������ƶ�������Ca5��PO4��3OH�ܽ⣬���ȣ�� 5Ca2+��aq��+3PO43-��aq��+OH-��aq�������ƶ�������Ca5��PO4��3OH�ܽ⣬���ȣ��
5Ca2+��aq��+3PO43-��aq��+OH-��aq�������ƶ�������Ca5��PO4��3OH�ܽ⣬���ȣ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ǻ���ʯ[Ca5(PO4)3OH]��һ��һ����Ҫ�����������ϡ��䳣�õ��Ʊ����������֣�
����A����Ũ��ˮ�ֱ��Ca(NO3)2��(NH4)2HPO4��Һ��pHԼΪ12���ھ��ҽ����£���(NH4)2HPO4��Һ��������Ca(NO3)2��Һ�С�
����B�����ҽ����£���H3PO4��Һ�����μӵ�Ca(OH)2����Һ�С�

3�ָ��ε��ܽ������ҺpH�ı仯����ͼ��ʾ��ͼ���������Ǹ�����Ũ�ȵĶ��������ش��������⣺
��1����ɷ���A�ͷ���B���Ʊ�Ca5(PO4)3OH�Ļ�ѧ��Ӧ����ʽ��
��5Ca(NO3)2��3(NH4)2HPO4��4NH3��H2O��Ca5(PO4)3OH���� ��
��5Ca(OH)2��3H3PO4��
��2���뷽��A��ȣ�����B���ŵ��� ��
��3������B�У����H3PO4��Һ�μӹ��죬�ƵõIJ��ﲻ������ԭ����
��
��4��ͼ����ʾ3�ָ��������������ȶ��Ĵ�����ʽ�� ���ѧʽ����
��5����մ���������ϣ���ø�������²����������ʣ������ȣ�ݡ���ϻ�ѧƽ���ƶ�ԭ����������ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ǻ���ʯ[Ca5(PO4)3OH]��һ����Ҫ�����������ϡ��䳣�õ��Ʊ����������֣�
����A����Ũ��ˮ�ֱ��Ca(NO3)2��(NH4)2HPO4��Һ��pHԼΪ12���ھ��ҽ����£���(NH4)2HPO4��Һ��������Ca(NO3)2��Һ�С�
����B�����ҽ����£���H3PO4��Һ�����μӵ�Ca(OH)2����Һ�С�
3�ָ��ε��ܽ������ҺpH�ı仯����ͼ��ʾ��ͼ���������Ǹ�����Ũ�ȵĶ��������ش��������⣺
(1)��ɷ���A�ͷ���B���Ʊ�Ca5(PO4)3OH�Ļ�ѧ��Ӧ����ʽ��
��5 Ca(NO3)2 + 3(NH4)2HPO4+ 4NH3��H2O = Ca5(PO4)3OH��+ + ��2�֣�
��5Ca(OH)2 + 3H3PO4 = ��2�֣�
(2)�뷽��A��ȣ�����B���ŵ��� ����3�֣�
(3)����B�У����H3PO4��Һ�μӹ��죬�ƵõIJ��ﲻ������ԭ���� ����3�֣�
(4)ͼ����ʾ3�ָ��������������ȶ��Ĵ�����ʽ�� ���ѧʽ������2�֣�
(5)��մ���������ϣ���ø�������²����������ʣ������ȣ�ݡ���ϻ�ѧƽ���ƶ�ԭ����������ԭ�� ����3�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ۻ�ѧ����ѡ��ѧ�뼼���ݣ�15�֣�
�ǻ���ʯ[Ca5��PO4��3OH]��һ��һ����Ҫ�����������ϡ��䳣�õ��Ʊ����������֣�
����A����Ũ��ˮ�ֱ��Ca��NO3��2�ͣ�NH4��2HPO4��Һ��pHԼΪ12���ھ��ҽ����£�����NH4��2HPO4��Һ��������Ca��NO3��2��Һ�С�
����B�����ҽ����£���H3PO4��Һ�����μӵ�Ca��OH��2����Һ�С�
3�ָ��ε��ܽ������ҺpH�ı仯����ͼ��ʾ��ͼ���������Ǹ�����Ũ�ȵĶ��������ش��������⣺
��1����ɷ���A�ͷ���B���Ʊ�Ca5��PO4��3OH�Ļ�ѧ��Ӧ����ʽ��
��5 Ca��NO3��2 + 3��NH4��2HPO4 + 4NH3��H2O = Ca5��PO4��3OH��+ + ��2�֣�
��5Ca��OH��2 + 3H3PO4 = ��2�֣�
��2���뷽��A��ȣ�����B���ŵ��� ����3�֣�
��3������B�У����H3PO4��Һ�μӹ��죬�ƵõIJ��ﲻ������ԭ����
����3�֣�
��4��ͼ����ʾ3�ָ��������������ȶ��Ĵ�����ʽ�� ���ѧʽ������2�֣�
��5����մ���������ϣ���ø�������²����������ʣ������ȣ�ݡ���ϻ�ѧƽ���ƶ�ԭ����������ԭ�� ����3�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ���Ͽ��Ը��������ۣ���ѧ���� ���ͣ������
�ǻ���ʯ[Ca5(PO4)3OH]��һ����Ҫ�����������ϡ��䳣�õ��Ʊ����������֣�
����A����Ũ��ˮ�ֱ��Ca(NO3)2��(NH4)2HPO4��Һ��pHԼΪ12���ھ��ҽ����£���(NH4)2HPO4��Һ��������Ca(NO3)2��Һ�С�
����B�����ҽ����£���H3PO4��Һ�����μӵ�Ca(OH)2����Һ�С�
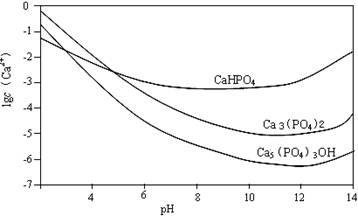
3�ָ��ε��ܽ������ҺpH�ı仯����ͼ��ʾ��ͼ���������Ǹ�����Ũ�ȵĶ��������ش��������⣺
(1)��ɷ���A�ͷ���B���Ʊ�Ca5(PO4)3OH�Ļ�ѧ��Ӧ����ʽ��
��5 Ca(NO3)2 + 3(NH4)2HPO4 + 4NH3��H2O = Ca5(PO4)3OH��+ + ��2�֣�
��5Ca(OH)2 + 3H3PO4 = ��2�֣�
(2)�뷽��A��ȣ�����B���ŵ��� ����3�֣�
(3)����B�У����H3PO4��Һ�μӹ��죬�ƵõIJ��ﲻ������ԭ���� ����3�֣�
(4)ͼ����ʾ3�ָ��������������ȶ��Ĵ�����ʽ�� ���ѧʽ������2�֣�
(5)��մ���������ϣ���ø�������²����������ʣ������ȣ�ݡ���ϻ�ѧƽ���ƶ�ԭ����������ԭ�� ����3�֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com