
”¾ĢāÄæ”æÓŠ»ś»ÆŗĻĪļFŹĒŅ»ÖÖÖŲŅŖµÄÓŠ»śŗĻ³ÉÖŠ¼äĢ壬ĘäŗĻ³ÉĀ·ĻßČēĻĀĶ¼ĖłŹ¾£ŗ

ŅŃÖŖ£ŗ¢ŁAµÄŗĖ“Ź²ÕńĒāĘ×Ķ¼ÖŠĻŌŹ¾Į½×é·å
¢ŚFµÄ½į¹¹¼ņŹ½ĪŖ£ŗ
¢ŪĶس£ŌŚĶ¬Ņ»øöĢ¼Ō×ÓÉĻĮ¬ÓŠĮ½øöōĒ»ł²»ĪČ¶Ø£¬Ņ×ĶŃĖ®ŠĪ³ÉōŹ»ł”£
¢Ü
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©AµÄĆū³ĘĪŖ______________£ØĻµĶ³ĆüĆū·Ø£©£»ZÖŠĖłŗ¬¹ŁÄÜĶŵÄĆū³ĘŹĒ___________”£
£Ø2£©·“Ó¦¢ńµÄ·“Ó¦Ģõ¼žŹĒ__________£»·“Ó¦¢óµÄ·“Ó¦ĄąŠĶĪŖ__________ ”£
£Ø3£©EµÄ½į¹¹¼ņŹ½ĪŖ_______________________”£
£Ø4£©Š“³ö·“Ó¦¢õµÄ»Æѧ·½³ĢŹ½____________________________________________”£
£Ø5£©WŹĒZµÄĶ¬ĻµĪļ£¬Ļą¶Ō·Ö×ÓÖŹĮæ±ČZ“ó14£¬ŌņWµÄĶ¬·ÖŅģ¹¹ĢåÖŠĀś×ćĻĀĮŠĢõ¼ž£ŗ
¢ŁÄÜ·¢ÉśŅų¾µ·“Ó¦£¬¢Ś±½»·ÉĻÓŠĮ½øöČ”“ś»ł£¬¢Ū²»ÄÜĖ®½ā£¬ÓöFeCl3ČÜŅŗ²»ĻŌÉ«µÄ½į¹¹¹²ÓŠ
_________Ö֣ز»°üĄØĮ¢ĢåŅģ¹¹£©£¬ŗĖ“Ź²ÕńĒāĘ×ÓŠĖÄ×é·åµÄ½į¹¹ĪŖ____________”£
£Ø6£©²ĪÕÕČēĻĀŗĻ³ÉĀ·Ļߣ¬Éč¼ĘŅŌ2”ŖĀȱūĶéĪŖĘšŹ¼ŌĮĻŗĻ³É±ūČ©µÄŗĻ³ÉĀ·ĻߣØĪŽ»śŹŌ¼ĮČĪŃ”£©_____________________________________________________________”£
”¾“š°ø”æ 2£¼×»ł£2£±ū“¼ ·ÓōĒ»ł”¢Č©»ł ÅØĮņĖį£¬¼ÓČČ Ńõ»Æ·“Ó¦ (CH3)2CHCOOH  6
6 ![]()

”¾½āĪö”æAµÄŗĖ“Ź²ÕńĒāĘ×Ķ¼ÖŠĻŌŹ¾Į½×é·å£¬½įŗĻAµÄ»ÆѧŹ½£¬AĪŖ £¬øł¾ŻBµÄ»ÆѧŹ½æÉÖŖ£¬A·¢ÉśōĒ»łµÄĻūČ„·“Ӧɜ³ÉB£¬ŌņBĪŖ
£¬øł¾ŻBµÄ»ÆѧŹ½æÉÖŖ£¬A·¢ÉśōĒ»łµÄĻūČ„·“Ӧɜ³ÉB£¬ŌņBĪŖ![]() £¬øł¾ŻŠÅĻ¢¢Ü£¬B·¢Éś¼Ó³É·“Ӧɜ³ÉC£¬CĪŖ
£¬øł¾ŻŠÅĻ¢¢Ü£¬B·¢Éś¼Ó³É·“Ӧɜ³ÉC£¬CĪŖ![]() £¬C“ß»ÆŃõ»ÆÉś³ÉD£¬DĪŖ(CH3)2CHCHO£¬DÓėĒāŃõ»ÆĶŠü×ĒŅŗ·“Ó¦ŗóĖį»ÆÉś³ÉE£¬EĪŖ(CH3)2CHCOOH£»øł¾ŻFµÄ½į¹¹
£¬C“ß»ÆŃõ»ÆÉś³ÉD£¬DĪŖ(CH3)2CHCHO£¬DÓėĒāŃõ»ÆĶŠü×ĒŅŗ·“Ó¦ŗóĖį»ÆÉś³ÉE£¬EĪŖ(CH3)2CHCOOH£»øł¾ŻFµÄ½į¹¹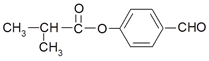 æÉÖŖ£¬ZĪŖ¶ŌōĒ»ł±½¼×Č©£¬Ņņ“ĖYĪŖ
æÉÖŖ£¬ZĪŖ¶ŌōĒ»ł±½¼×Č©£¬Ņņ“ĖYĪŖ![]() £¬XĪŖ
£¬XĪŖ![]() ”£
ӣ
(1)AĪŖ £¬Ćū³ĘĪŖ2£¼×»ł£2£±ū“¼£»ZĪŖ¶ŌōĒ»ł±½¼×Č©£¬Ėłŗ¬¹ŁÄÜĶÅÓŠ·ÓōĒ»ł”¢Č©»ł£¬¹Ź“š°øĪŖ£ŗ2£¼×»ł£2£±ū“¼£»·ÓōĒ»ł”¢Č©»ł£»
£¬Ćū³ĘĪŖ2£¼×»ł£2£±ū“¼£»ZĪŖ¶ŌōĒ»ł±½¼×Č©£¬Ėłŗ¬¹ŁÄÜĶÅÓŠ·ÓōĒ»ł”¢Č©»ł£¬¹Ź“š°øĪŖ£ŗ2£¼×»ł£2£±ū“¼£»·ÓōĒ»ł”¢Č©»ł£»
(2)·“Ó¦¢ńĪŖōĒ»łµÄĻūČ„·“Ó¦£¬·“Ó¦Ģõ¼žĪŖÅØĮņĖį£¬¼ÓČČ£»·“Ó¦¢óŹĒ“¼µÄ“ß»ÆŃõ»Æ£¬¹Ź“š°øĪŖ£ŗÅØĮņĖį£¬¼ÓČČ£»Ńõ»Æ·“Ó¦£»
(3)øł¾ŻÉĻŹö·ÖĪö£¬EĪŖ(CH3)2CHCOOH£¬¹Ź“š°øĪŖ£ŗ(CH3)2CHCOOH£»
(4)·“Ó¦¢õĪŖ±½»·²ąĮ“µÄČ”“ś·“Ó¦£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ £»
£»
(5)WŹĒZ(¶ŌōĒ»ł±½¼×Č©)µÄĶ¬ĻµĪļ£¬Ļą¶Ō·Ö×ÓÖŹĮæ±ČZ“ó14£¬¢ŁÄÜ·¢ÉśŅų¾µ·“Ó¦£¬ĖµĆ÷ŗ¬ÓŠČ©»ł£»¢Ś±½»·ÉĻÓŠĮ½øöČ”“ś»ł£¬¢Ū²»ÄÜĖ®½ā£¬ÓöFeCl3ČÜŅŗ²»ĻŌÉ«£¬ĖµĆ÷ƻӊ·ÓōĒ»łŗĶõ„»ł£¬Āś×ćĢõ¼žµÄÓŠ£ŗ±½»·ÉĻŗ¬ÓŠ1øöČ©»łŗĶ1øö”ŖCH2OH£¬ÓŠ3ÖÖ½į¹¹£»±½»·ÉĻŗ¬ÓŠ1øöČ©»łŗĶ1øö”ŖO CH3£¬ÓŠ3ÖÖ½į¹¹£»¹²6ÖÖ£»ĘäÖŠŗĖ“Ź²ÕńĒāĘ×ÓŠĖÄ×é·åµÄ½į¹¹ĪŖ![]() £¬¹Ź“š°øĪŖ£ŗ6£»
£¬¹Ź“š°øĪŖ£ŗ6£»![]() £»
£»
(6)ŅŌ2”ŖĀȱūĶéĪŖŌĮĻŗĻ³É±ūČ©(CH3CH2CHO)£¬Ö»ŠčŅŖ½«ĀČŌ×ÓĻūČ„£¬Č»ŗóøł¾ŻŠÅĻ¢¢ÜŌŚĢ¼Ģ¼Ė«¼üÖŠŅżČėōĒ»ł£¬ŌŁŃõ»Æ¼“æÉ£¬ŗĻ³ÉĀ·ĻßĪŖ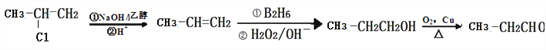 £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ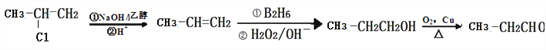 ”£
ӣ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ¹ŲÓŚ¹č¼°Ęä»ÆŗĻĪļµÄĖµ·ØÖŠ£¬ÕżČ·µÄŹĒ£Ø £©
A.¹čŹĒ³£ÓƵİėµ¼Ģå²ÄĮĻ£¬æÉÓĆÓŚÖĘŌģ¹āµ¼ĻĖĪ¬B.¶žŃõ»Æ¹čŹĒĖįŠŌŃõ»ÆĪļ£¬¹Ź²»ÓėČĪŗĪĖį·“Ó¦
C.¹čĖįŹĒŅ»ÖÖ¶žŌŖČõĖį£¬ĘäĖįŠŌĒæÓŚĢ¼ĖįD.ÖĘŌģĘÕĶز£Į§µÄÖ÷ŅŖŌĮĻŹĒ“æ¼ī”¢ŹÆ»ŅŹÆŗĶŹÆÓ¢
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æPOCl3¹ć·ŗÓĆÓŚČ¾ĮĻµČ¹¤Ņµ”£Ä³»ÆѧѧĻ°Š”×é½čÖśĄĶßĪżŃŠ¾ææÕĘų³É·ÖµÄĒś¾±źµ£Ø×°ÖĆ¼×£©ŗĻ³ÉPC13£¬²¢²ÉČ”PCl3Ńõ»Æ·ØÖʱøPOCl3”£
ŅŃÖŖ£ŗ£Ø1£©PCl3µÄČŪµćĪŖ-112”ę£¬·ŠµćĪŖ75.5”ę£¬ÓöĖ®Éś³ÉH3PO3ŗĶHCl£»
£Ø2£©2PCl3+O2==2POCl3”£
”¾ŹµŃé¢ń”æÖʱøPCl3
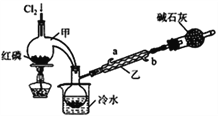
£Ø1£©ŹµŃéŹŅÖʱøCl2µÄŌĄķŹĒ________________________”£
£Ø2£©¼īŹÆ»ŅµÄ×÷ÓĆ³żĮĖ“¦ĄķĪ²ĘųĶā»¹ÓŠ________________________”£
£Ø3£©×°ÖĆŅŅÖŠĄäÄżĖ®“Ó_____£ØŃ”Ģīa»ņb£©½ųČė”£
”¾ŹµŃé¢ņ”æÖʱøPOCl3
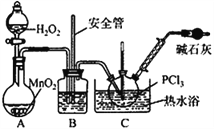
£Ø4£©ŹµŃéŹŅ³£ÓĆÓŠĪ¢æ׵ďŌ¼ĮĘæ±£“ęH2O2£¬”°Ī¢æ×”±ÓėÉĻŹö×°ÖĆÖŠµÄ___________£ØŅĒĘ÷Ćū³Ę£©ÄæµÄŹĒŅ»ÖĀµÄ”£
£Ø5£©CÖŠ·“Ó¦ĪĀ¶ČæŲÖĘŌŚ60~65”ę£¬ĘäŌŅņŹĒ________________________”£
”¾ŹµŃé¢ó”æ²ā¶ØPOCl3ŗ¬Įæ
¢Ł×¼Č·³ĘČ”30.70gPOC13²śĘ·£¬ÖĆÓŚŹ¢ÓŠ60.00mLÕōĮóĖ®µÄĖ®½āĘæÖŠŅ”¶ÆÖĮĶźČ«Ė®½ā£»
¢Ś½«Ė®½āŅŗÅä³É100.00mLČÜŅŗ£¬Č”10.00mLČÜŅŗӌ׶ŠĪĘæÖŠ£»
¢Ū¼ÓČė10.00mL3.200mol/LAgNO3±ź×¼ČÜŅŗ£¬²¢¼ÓČėÉŁŠķĻõ»ł±½ÓĆĮ¦Ņ”¶Æ£¬Ź¹³Įµķ±ķĆę±»ÓŠ»śĪļø²øĒ£»
¢ÜŅŌFe3+ ĪŖÖøŹ¾¼Į£¬ÓĆ0.2000mol/LKSCN ČÜŅŗµĪ¶Ø¹żĮæµÄAgNO3ČÜŅŗ£¬“ļµ½µĪ¶ØÖÕµćŹ±¹²ÓĆČ„10.00 mL KSCN ČÜŅŗ”£
ŅŃÖŖ£ŗAg++SCN-==AgSCN”ż Ksp(AgCl)>Ksp(AgSCN )”£
£Ø6£©POC13Ė®½āµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ________________________”£
£Ø7£©µĪ¶ØÖÕµćµÄĻÖĻóĪŖ____________£¬ÓĆĻõ»ł±½ø²øĒ³ĮµķµÄÄæµÄŹĒ________________________”£
£Ø8£©·“Ó¦ÖŠPOC13µÄ°Ł·Öŗ¬ĮæĪŖ________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£Ø1£©ĻĀĮŠŅĒĘ÷Ź¹ÓĆĒ°±ŲŠė¼ģ²éŹĒ·ńĀ©Ė®µÄÓŠ_________(ĢīŠņŗÅ)”£
A£®ČŻĮæĘæ B£®ÕōĮóÉÕĘæ C£®·ÖŅŗĀ©¶· D£®ĮæĶ² E£®Õō·¢Ćó
£Ø2£©ŹµŃéŹŅÓĆNa2CO3”¤10H2O¾§ĢåÅäÖĘ0.5mol/LµÄNa2CO3ČÜŅŗ970mL£¬Ó¦Ń”ÓƵÄČŻĮæĘæµÄ¹ęøń___________£¬Ó¦³ĘĮæ¶ąÉŁæĖNa2CO3”¤10H2O¾§Ģå_____”£
£Ø3£©Ä³Ń§ÉśÓūÓĆ10mol”¤L£1ÅØŃĪĖįŗĶÕōĮóĖ®ÅäÖĘ500mLĪļÖŹµÄĮæÅضČĪŖ5mol”¤L£1µÄĻ”ŃĪĖį”£ŌņĖłŠčŅŖÅØŃĪĖįĢå»żĪŖ___mL£¬ÅäÖĘ¹ż³ĢÖŠÕżČ·µÄ²Ł×÷Ė³ŠņŹĒ£Ø×ÖÄø±ķŹ¾£¬Ćæøö×ÖÄøÖ»ÄÜÓĆŅ»“Ī£©_________
A.ÓĆÉŁĮæĖ®Ļ“µÓÉÕ±2”«3“Ī£¬Ļ“µÓŅŗ¾ł×¢ČėČŻĮæĘ棬Õńµ“
B.ÓĆĮæĶ²ĮæČ”ĖłŠčÅØŃĪĖį£¬ŃŲ²£Į§°ōµ¹ČėÉÕ±ÖŠ£¬ŌŁ¼ÓČėÉŁĮæĖ®£¬ÓĆ²£Į§°ōĀżĀż½Į¶Æ£¬Ź¹Ęä»ģŗĻ¾łŌČ
C.½«ŅŃĄäČ“µÄŃĪĖįŃŲ²£Į§°ō×¢Čė500mLČŻĮæĘæÖŠ
D.½«ČŻĮæĘæøĒ½ō£¬Õńµ“£¬Ņ”ŌČ
E.øÄÓĆ½ŗĶ·µĪ¹Ü¼ÓĖ®£¬Ź¹ČÜŅŗ°¼ĆęĒ”ŗĆÓėæĢ¶ČĻąĒŠ
F.¼ĢŠųĶłČŻĮæĘæÄŚŠ”ŠÄ¼ÓĖ®£¬Ö±µ½ŅŗĆę½Ó½üæĢ¶Č1”«2cm“¦
£Ø4£©ÅäÖĘŅ»¶ØĢå»ż”¢Ņ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ,ŹµŃé½į¹ūĘ«øßÓ°ĻģµÄŹĒ_______
A.ČŻĮæĘæÖŠŌÓŠÉŁĮæĖ® B.ČܽāĖłÓĆÉÕ±Ī“Ļ“µÓ C.¶ØČŻŹ±ŃöŹÓ¹Ū²ģæĢ¶ČĻß D.¶ØČŻŹ±ø©ŹÓ¹Ū²ģæĢ¶ČĻß
£Ø5£©ĻĀĶ¼ŹĒijĶ¬Ń§ŌŚŹµŃéŹŅÅäÖĘøĆNaClČÜŅŗµÄ¹ż³ĢŹ¾ŅāĶ¼£¬ĘäÖŠÓŠ“ķĪóµÄŹĒ______(Ģī²Ł×÷ŠņŗÅ)”£


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻą¶Ō·Ö×ÓÖŹĮæĪŖMµÄĘųĢ¬»ÆŗĻĪļV L(±ź×¼×“æö)£¬ČÜÓŚm gĖ®ÖŠ£¬µĆµ½ÖŹĮæ·ÖŹżĪŖwµÄČÜŅŗ£¬ĪļÖŹµÄĮæÅضČĪŖc mol”¤L£1£¬ĆܶČĪŖ¦Ń g”¤cm£3”£ŌņĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ(””””)
A. Ļą¶Ō·Ö×ÓÖŹĮæM£½22.4mw/(1”Ŗw)V B. ĪļÖŹµÄĮæÅضČc£½1000¦ŃV/(MV+22.4m)
C. ČÜŅŗµÄÖŹĮæ·ÖŹżw£½MV/22.4m D. ČÜŅŗĆÜ¶Č¦Ń£½cM/1000w
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŹĄ½ēĪĄÉś×éÖÆ£ØWHO£©½«ClO2ĮŠĪŖA¼¶øߊ§°²Č«Ćš¾śĻū¶¾¼Į£¬ĖüŌŚŹ³Ę·±£ĻŹ”¢ŅūÓĆĖ®Ļū¶¾µČ·½ĆęÓŠ¹ć·ŗÓ¦ÓĆ”£ClO2ŹōÓŚ
A.ĖįB.¼īC.Ńõ»ÆĪļD.ŃĪ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ(1)ĻĀĮŠĖÄÖÖĪļÖŹ£¬¢ŁNAøöHCl ¢Ś24gCH4 ¢Ū4.816”Į1023øöH2O2·Ö×Ó ¢Ü10.8mLĖ®£ØĆܶČĪŖ1g”¤cm£3£©”£
¢”£®ĖÄÖÖĪļÖŹĖłŗ¬·Ö×ÓŹżÓɶąµ½ÉŁµÄĖ³ŠņŹĒ£ŗ_________________________________£»
¢¢£®ĖÄÖÖĪļÖŹĖłŗ¬Ō×ÓŹżÓɶąµ½ÉŁµÄĖ³ŠņŹĒ£ŗ_________________________________£»
¢££®ĖÄÖÖĪļÖŹĖłŗ¬HÓɶąµ½ÉŁµÄĖ³ŠņŹĒ£ŗ_____________________________________£»
¢¤£®ĖÄÖÖĪļÖŹµÄÖŹĮæÓɶąµ½ÉŁµÄĖ³ŠņŹĒ£ŗ_____________________________________”£
(2)H2OÓėCO2µÄÖŹĮæ±ČĪŖ18:22£¬ŌņH2OŗĶCO2µÄĪļÖŹµÄĮæÖ®±ČĪŖ________£¬·Ö×ÓøöŹż±ČĪŖ_______£¬Ō×ÓøöŹż±ČĪŖ__________£¬Ėłŗ¬ŃõŌ×ÓøöŹż±ČĪŖ___________”£
(3)ŌŚ±ź×¼×“æöĻĀ£¬µČÖŹĮæµÄO3ŗĶCO2±Č½Ļ£¬ĆܶȱČĪŖ________£¬·Ö×ÓŹżÖ®±ČĪŖ_________£¬Ō×ÓŹżÖ®±Č______£¬Ģå»ż±ČĪŖ_______£¬ĪļÖŹµÄĮæÖ®±Č__________”£
(4)CH4ÓėH2µÄ»ģŗĻĘųĢ壬ĘäÖŹĮæ°Ł·Öŗ¬Įæ·Ö±šĪŖ80%”¢20%£¬Ōņ»ģŗĻĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæĪŖ__________”£
(5)±ź×¼×“æöĻĀ£¬Ä³ĘųĢåĆܶČĪŖ1.96g/L£¬ŌņøĆĘųĢåµÄĦ¶ūÖŹĮæĪŖ_____£Ø±£ĮōÕūŹż£©
(6)°Ń100æĖijNaOHČÜŅŗ£ØĆܶȏĒ1.22g/cm3£©Õō·¢ÅØĖõ£¬ÓąĻĀČÜŅŗ50mlŹ±£¬ĪļÖŹµÄĮæÅØ¶ČŹĒ8mol/L£¬ŌČÜŅŗµÄĪļÖŹµÄĮæÅØ¶ČŹĒ _________________”£
(7)ŅŃÖŖNa2S”¢Na2SO3”¢Na2SO4»ģŗĻĪļÖŠĮņŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖa%£¬ŌņŃõŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ_____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”涔±½ĢŖŹĒĪŅ¹ś×ŌÖ÷ŃŠ·¢µÄŅ»ĄąÓĆÓŚÖĪĮĘ¼±ŠŌȱŃŖŠŌÄŌ×äµÄŠĀŅ©”£ŗĻ³É¶”±½ĢŖ(J)µÄŅ»ÖÖĀ·ĻßČēĻĀ£ŗ


£Ø1£©AµÄĆū³ĘŹĒ_______________£¬A·Ö×ÓÖŠ×ī¶ąÓŠ_____øöŌ×Ó¹²Ę½Ćę”£
£Ø2£©BÉś³ÉAµÄ»Æѧ·½³ĢŹ½______________________”£
£Ø3£©DÉś³ÉEµÄ·“Ó¦ĄąŠĶĪŖ_________£¬ŹŌ¼ĮaŹĒ_________”£
£Ø4£©FµÄ½į¹¹¼ņŹ½_____________________”£
£Ø5£©JŹĒŅ»ÖÖõ„£¬·Ö×ÓÖŠ³ż±½»·Ķā»¹ŗ¬ÓŠŅ»øöĪåŌŖ»·”£Š“³öHÉś³ÉJµÄ»Æѧ·½³ĢŹ½_____£Ø×¢Ć÷·“Ó¦Ģõ¼ž£©”£
£Ø6£©![]() £¬XµÄĶ¬·ÖŅģ¹¹ĢåÖŠ£ŗ¢ŁÄÜ·¢ÉśŅų¾µ·“Ó¦£»¢ŚÄÜÓėĀČ»ÆĢśČÜŅŗ·¢ÉśĻŌÉ«·“Ó¦”£Āś×ćÉĻŹöĢõ¼žµÄXµÄĶ¬·ÖŅģ¹¹Ģå¹²ÓŠ______ÖÖ£¬Š“³öĘäÖŠŗĖ“Ź²ÕńĒāĘ×ÓŠĪå×éĪüŹÕ·åµÄ½į¹¹¼ņŹ½___________________”£
£¬XµÄĶ¬·ÖŅģ¹¹ĢåÖŠ£ŗ¢ŁÄÜ·¢ÉśŅų¾µ·“Ó¦£»¢ŚÄÜÓėĀČ»ÆĢśČÜŅŗ·¢ÉśĻŌÉ«·“Ó¦”£Āś×ćÉĻŹöĢõ¼žµÄXµÄĶ¬·ÖŅģ¹¹Ģå¹²ÓŠ______ÖÖ£¬Š“³öĘäÖŠŗĖ“Ź²ÕńĒāĘ×ÓŠĪå×éĪüŹÕ·åµÄ½į¹¹¼ņŹ½___________________”£
£Ø7£©ĄūÓĆĢāÖŠŠÅĻ¢ŗĶĖłŃ§ÖŖŹ¶£¬Š“³öŅŌ¼×ĶéŗĶ»ÆŗĻĪļDĪŖŌĮĻ£¬ŗĻ³É ![]() µÄĀ·ĻßĮ÷³ĢĶ¼____________________£ØĘäĖüŹŌ¼Į×ŌŃ”£©”£
µÄĀ·ĻßĮ÷³ĢĶ¼____________________£ØĘäĖüŹŌ¼Į×ŌŃ”£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æTiO2ŹĒŅ»ÖÖŠŌÄÜÓÅĮ¼µÄ°ėµ¼Ģå¹ā“߻ƼĮ£¬ÄÜÓŠŠ§µŲĪüø½ÓŠ»śĪŪČ¾Īļ£ØČē¼×Č©”¢¼×±½µČ)ŗĶŗ¬µŖ»ÆŗĻĪļ(ČēNH3”¢CN£µČ£©×Ŗ»ÆĪŖCO2ŗĶN2µČŠ”·Ö×ÓĪļÖŹ”£
£Ø1£©Ti»łĢ¬ŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ________________”£
£Ø2£©¼×Č©HCHO·Ö×Óæռ乹ŠĶĪŖ_____£»·Ö×ÓÖŠĢ¼Ō×Ó¹ģµĄŌÓ»ÆĄąŠĶĪŖ_____£¬¦Š¼üŗĶ¦Ņ¼üµÄøöŹżÖ®±ČĪŖ____£¬
£Ø3£©°±Ęų¼«Ņ×ČÜÓŚĖ®£¬ŹĒŅņĪŖ°±ŗĶĖ®µÄ·Ö×Ó¾łŹĒ_________£¬»¹ŅņĪŖ___________”£
£Ø4£©¼×±½·Ö×ÓÖŠÄܹ»¹²Ę½ĆęµÄŌ×Ó×ī¶ąĪŖ____øö£»±½»·²»Ņ×±»Ā±ĖŲ¼Ó³É£¬¶ų±Č½ĻČŻŅ×±»Ā±ĖŲČ”“ś±½»·ÉĻµÄĒā£¬ŌŅņŹĒ___________________”£
£Ø5£©ŗ¬CN£µÄĪŪĖ®¶¾ŠŌ¼«“ó£¬ÓĆNaClOĻČ°ŃCN£Ńõ»ÆĪŖCNO££¬Č»ŗóŌŚĖįŠŌĢõ¼žĻĀŌŁ½«CNO£Ńõ»ÆĪŖĪŽĪŪČ¾µÄĘųĢ唣ĒėŠ“³öÓėCNO£»„ĪŖµČµē×ÓĢåµÄĪ¢Į£______·Ö×Ó»ņĄė×Ó£¬Š“Ņ»ÖÖ£©”£
£Ø6£©Ti[(CN)4]2-ÖŠTi2+ÓėCN-µÄCŌ×ÓŠĪ³ÉÅäĪ»¼ü”£²»æ¼ĀĒæռ乹ŠĶ£¬Ti[(CN)4]2-µÄ½į¹¹æɱķŹ¾ĪŖ_____________________”£
£Ø7£©TiµÄijŃõ»ÆĪļŗĶCaOĻą»„×÷ÓĆÄÜŠĪ³ÉīŃĖįŃĪµÄ¾§°ū½į¹¹ČēĶ¼ĖłŹ¾£ØTi4+Ī»ÓŚĮ¢·½ĢåµÄ¶„µć£¬Ca2+ “¦ÓŚĮ¢·½ĢåµÄÖŠŠÄ£©”£øĆ¾§ĢåÖŠ£¬Ti4+ŗĶÖÜĪ§____ øöO2-Ļą½ōĮŚ£»ČōøĆ¾§°ūµÄĆܶČĪŖdg/cm3ŌņīŃŃõ¼üµÄ¼ü³¤ĪŖ______pm £ØÓĆ“ųNAµÄ“śŹżŹ½±ķŹ¾£©”£

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com