
”¾ĢāÄæ”æ²ŻĖį¾§ĢåµÄ×é³ÉæÉÓĆH2C2O4”¤xH2O±ķŹ¾£¬ĪŖĮĖ²ā¶ØxÖµ£¬½ųŠŠČēĻĀŹµŃé£ŗ³ĘČ”Wg²ŻĖį¾§Ģ壬Åä³É100mLĖ®ČÜŅŗ”£ĮæČ”25.00mLĖłÅäÖĘµÄ²ŻĖįČÜŅŗÖĆӌ׶ŠĪĘæÄŚ£¬¼ÓČėŹŹĮæĻ”H2SO4ŗó£¬ÓĆÅضČĪŖamol”¤L-1µÄKMnO4ČÜŅŗµĪ¶Ø£¬Ėł·¢ÉśµÄ·“Ó¦ĪŖ£ŗKMnO4£«H2C2O4£«H2SO4 -K2SO4£«CO2”ü£«MnSO4£«H2O£ØĪ“ÅäĘ½£©
ŹŌ»Ų“š£ŗ
£Ø1£©ŹµŃéÖŠ£¬KMnO4ČÜŅŗӦװŌŚ___________Ź½µĪ¶Ø¹ÜÖŠ”£
£Ø2£©µĪ¶Ø¹ż³ĢÖŠŠčŅŖ¼ÓČėµÄÖøŹ¾¼ĮĪŖ___________(ĢīÖøŹ¾¼ĮµÄĆū³Ę»ņ”°²»ŠčŅŖ”±)£¬Č·¶Ø·“Ó¦“ļµ½µĪ¶ØÖÕµćŹ±µÄĻÖĻóŹĒ ”£
£Ø3£©Ķ¼I±ķŹ¾100mLĮæĶ²ÖŠŅŗĆęµÄĪ»ÖĆ£¬AÓėB£¬BÓėC£¬æĢ¶Č¼ä¾łĻą²ī10mL£¬Čē¹ūæĢ¶ČAĪŖ30£¬ĮæĶ²ÖŠŅŗĢåµÄĢå»żŹĒ________mL”£Ķ¼II±ķŹ¾25mLµĪ¶Ø¹ÜÖŠŅŗĆęµÄĪ»ÖĆ£¬DÓėEæĢ¶Č¼äŅ²Ļą²ī10mL£¬Čē¹ūD“¦µÄ¶ĮŹżŹĒ5£¬ŌņµĪ¶Ø¹ÜÖŠŅŗĢåµÄ¶ĮŹżŹĒ________mL”£

£Ø4£©ŅŌĻĀ²Ł×÷Ōģ³É²ā¶Ø½į¹ūĘ«øßµÄŌŅņæÉÄÜŹĒ ”££ØÓĆ·ūŗÅĢīŠ“£©
A£®µĪ¶ØĒ°µĪ¶Ø¹ÜÓŠĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§
B£®µĪ¶ØÖÕµć¶ĮŹżŹ±£¬ø©ŹÓµĪ¶Ø¹ÜµÄæĢ¶Č£¬ĘäĖü²Ł×÷¾łÕżČ·
C£®Ź¢×°Ī“ÖŖŅŗµÄ׶ŠĪĘæÓĆÕōĮóĖ®Ļ“¹ż£¬Ī“ÓĆ“ż²āŅŗČóĻ“
D£®µĪ¶Øµ½ÖÕµć¶ĮŹżŹ±·¢ĻÖµĪ¶Ø¹Ü¼ā×ģ“¦Šü¹ŅŅ»µĪČÜŅŗ
E£®Ī“ÓƱź×¼ŅŗČóĻ“¼īŹ½µĪ¶Ø¹Ü
£Ø5£©ŌŚµĪ¶Ø¹ż³ĢÖŠČōÓĆa mol”¤L-1µÄKMnO4ČÜŅŗV mL£¬ŌņĖłÅäÖĘµÄ²ŻĖįČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ____________mol”¤L-1£¬ÓÉ“ĖæɼĘĖćxµÄÖµŹĒ ”£
”¾“š°ø”æ£Ø1£©Ėį £»
£Ø2£©²»ŠčŅŖ£»ČÜŅŗÓÉĪŽÉ«±äŗģÉ«£Ø×ĻŗģÉ«£©£¬ĒŅ°ė·ÖÖÓ²»ĶŹÉ«”£
£Ø3£©22.0 £¬ 5.60
£Ø4£©ADE
£Ø5£©av/10 £» (50w/9av)-5
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ
£Ø1£©øßĆĢĖį¼ŲČÜŅŗÓŠĒæŃõ»ÆŠŌ£¬Ó¦Ź¢×°ŌŚĖįŹ½µĪ¶Ø¹ÜÖŠ£»£Ø2£©KMnO4ČÜŅŗ³Ź×ĻÉ«£¬²ŻĖį·“Ó¦Ķź±Ļ£¬µĪČė×īŗóŅ»µĪKMnO4ČÜŅŗ£¬×ĻÉ«²»ĶŹČ„£¬ĖµĆ÷µĪ¶Øµ½ÖÕµć£¬²»ŠčŅŖĶā¼ÓÖøŹ¾¼Į£»ÓÉÓŚĖįŠŌøßĆĢĖį¼ŲČÜŅŗĻŌ×ĻŗģÉ«£¬ĖłŅŌÖÕµćŹ±µÄĻÖĻóŹĒČÜŅŗÓÉĪŽÉ«±äĪŖ×ĻŗģÉ«£¬ĒŅ°ė·ÖÖÓ²»ĶŹÉ«”£
£Ø3£©ÓÉÓŚĮæĶ²×ŌÉĻ¶ųĻĀæĢ¶ČŹĒÖš½„¼õŠ”µÄ£¬ĒŅĮæĶ²µÄ¾«Č·¶ČĪŖ0.1mL£¬ĖłŅŌøł¾ŻŅŗĆęµÄĪ»ÖĆæÉÖŖ£¬“ĖŹ±µÄ¶ĮŹżŹĒ22.0mL£»µĪ¶Ø¹ÜµÄæĢ¶Č×ŌÉĻ¶ųĻĀŹĒÖš½„Ōö“óµÄ£¬ĒŅµĪ¶Ø¹ÜµÄ¾«Č·¶ČĪŖ0.01mL£¬ĖłŅŌøł¾ŻŅŗĆęµÄĪ»ÖĆæÉÖŖ£¬“ĖŹ±µÄ¶ĮŹżŹĒ5.60mL£»
£Ø4£©A£®µĪ¶ØĒ°µĪ¶Ø¹ÜÓŠĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§£¬µ¼ÖĀ±ź×¼ŅŗĢå»żĘ«“ó£¬ŌņŌģ³É²ā¶Ø½į¹ūĘ«øߣ»B£®µĪ¶ØÖÕµć¶ĮŹżŹ±£¬ø©ŹÓµĪ¶Ø¹ÜµÄæĢ¶Č£¬ĘäĖü²Ł×÷¾łÕżČ·£¬µ¼ÖĀ±ź×¼ŅŗĢå»żĘ«Š”£¬ŌņŌģ³É²ā¶Ø½į¹ūĘ«µĶ£»C£®Ź¢×°Ī“ÖŖŅŗµÄ׶ŠĪĘæÓĆÕōĮóĖ®Ļ“¹ż£¬Ī“ÓĆ“ż²āŅŗČóĻ“£¬¶ŌŹµŃé²»Ó°Ļģ£»D£®µĪ¶Øµ½ÖÕµć¶ĮŹżŹ±·¢ĻÖµĪ¶Ø¹Ü¼ā×ģ“¦Šü¹ŅŅ»µĪČÜŅŗ£¬µ¼ÖĀ±ź×¼ŅŗĢå»żĘ«“ó£¬ŌņŌģ³É²ā¶Ø½į¹ūĘ«øߣ»E£®Ī“ÓƱź×¼ŅŗČóĻ“¼īŹ½µĪ¶Ø¹Ü£¬Ļąµ±ÓŚĻ”ŹĶ±ź×¼Ņŗ£¬ĖłŅŌĻūŗıź×¼ŅŗµÄĢå»żŌö¼Ó£¬²ā¶Ø½į¹ūĘ«øß”£¹ŹŃ”ADE”£
£Ø5£©øł¾Ż·“Ó¦µÄ·½³ĢŹ½æÉÖŖ£¬2KMnO4”«5H2C2O4£¬ŌņÓŠ²ŻĖįµÄÅضČ=(aV”Į10-3”Į![]() ”Į
”Į![]() )”Ā0.1=0.1aV mol/L”£
)”Ā0.1=0.1aV mol/L”£
Wg²ŻĖįµÄĪļÖŹµÄĮæ=aV”Į10-3”Į![]() ”Į
”Į![]() =0.01aVmol£¬ŌņH2C2O4”¤xH2OµÄĦ¶ūÖŹĮæĪŖWg”Ā0.01aVmol=
=0.01aVmol£¬ŌņH2C2O4”¤xH2OµÄĦ¶ūÖŹĮæĪŖWg”Ā0.01aVmol= ![]() g/mol”£ŌņÓŠ90+18x=
g/mol”£ŌņÓŠ90+18x=![]() ”£æɼĘĖćxµÄÖµŹĒ
”£æɼĘĖćxµÄÖµŹĒ![]() £5”£
£5”£


 ¶į¹Ś½š¾ķČ«ÄÜĮ·æ¼ĻµĮŠ“š°ø
¶į¹Ś½š¾ķČ«ÄÜĮ·æ¼ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĶ¼ÖŠµÄĆæŅ»øö·½øń±ķŹ¾ÓŠ¹ŲµÄŅ»ÖÖ·“Ó¦Īļ»ņÉś³ÉĪļ£¬ĘäÖŠXĪŖÕżŃĪ£¬A”¢C”¢D¾łĪŖĪŽÉ«ĘųĢ壬ĒŅĘųĢåCµÄĖ®ČÜŅŗĻŌ¼īŠŌ”£

(1)ÓĆ»ÆѧÓĆÓļ°“ŅŖĒóĢīæÕ
XµÄ»ÆѧŹ½ĪŖ£ŗ____£¬XÖŠŗ¬ÓŠ»Æѧ¼üĄąŠĶĪŖ_______£»AµÄµē×ÓŹ½£ŗ____£»GµÄ»ÆѧŹ½ĪŖ_________”£
(2)Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½________________”£
(3)Š“³ö·“Ó¦¢ŚµÄĄė×Ó·½³ĢŹ½________________”£
(4)¹żĮæµÄFe·ŪÓėGµÄĻ”ČÜŅŗ·“Ó¦£¬Ļņ·“Ó¦ŗóČÜŅŗÖŠ¼ÓČė¼īČÜŅŗ£¬ĻÖĻóŹĒ_________£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ
_________________________£»________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÓĆĻĀĶ¼ĖłŹ¾ŹµŃé×°ÖĆ£Ø¼Š³ÖŅĒĘ÷¼ŗĀŌČ„£©Ģ½¾æĶĖæÓė¹żĮæÅØĮņĖįµÄ·“Ó¦”£ĻĀĮŠŹµŃé²»ŗĻĄķµÄŹĒ( )

A. ¢ŚÖŠŃ”ÓĆĘ·ŗģČÜŅŗŃéÖ¤SO2µÄÉś³É
B. ¢ŪÖŠŃ”ÓĆNaOHČÜŅŗĪüŹÕ¶ąÓąµÄSO2
C. ĪŖČ·ČĻÓŠCuSO4Éś³É£¬Ļņ¢ŁÖŠ¼ÓĖ®£¬¹Ū²ģŃÕÉ«
D. ÉĻĻĀŅĘ¶Æ¢ŁÖŠĶĖææÉæŲÖĘSO2µÄ²śÉśÓėĶ£Ö¹
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĪ¢Į£²»¾ßÓŠ»¹ŌŠŌµÄŹĒ( )
A. H2 B. H£« C. Na D. CO
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æNH4Al(SO4)2ŹĒŹ³Ę·¼Ó¹¤ÖŠ×īĪŖæģ½ŻµÄŹ³Ę·Ģķ¼Ó¼Į£¬ÓĆÓŚ±ŗæ¾Ź³Ę·£»NH4HSO4ŌŚ·ÖĪöŹŌ¼Į”¢Ņ½Ņ©”¢µē×Ó¹¤ŅµÖŠÓĆĶ¾¹ć·ŗ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)NH4Al(SO4)2æÉ×÷¾»Ė®¼Į£¬ĘäŌĄķŹĒ____________________(ÓĆĄė×Ó·½³ĢŹ½ĖµĆ÷)”£
(2)ĻąĶ¬Ģõ¼žĻĀ£¬0.1 mol”¤L£1 NH4Al(SO4)2 ČÜŅŗÖŠ c(NH4+)________(Ģī”°µČÓŚ”±”¢”°“óÓŚ”±»ņ”°Š”ÓŚ”±)0.1 mol”¤L£1 NH4HSO4ČÜŅŗÖŠ c(NH4+)”£
(3)¾łĪŖ 0.1 mol”¤L£1 µÄ¼øÖÖµē½āÖŹČÜŅŗµÄpH ĖęĪĀ¶Č±ä»ÆµÄĒśĻßČēĻĀĶ¼1ĖłŹ¾”£
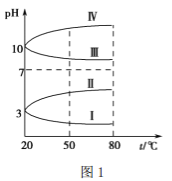
¢ŁĘäÖŠ·ūŗĻ0.1 mol”¤L£1 NH4Al(SO4)2ČÜŅŗµÄpHĖęĪĀ¶Č±ä»ÆµÄĒśĻߏĒ________(ĢīĀŽĀķŹż×Ö)£¬µ¼ÖĀ NH4Al(SO4)2ČÜŅŗµÄpH ĖęĪĀ¶Č±ä»ÆµÄŌŅņŹĒ_____________________£»
¢Ś20 ”ꏱ£¬0.1 mol”¤L£1 NH4Al(SO4)2ČÜŅŗÖŠ 2c(SO42£)£c(NH4+)£3c(Al3+)£½____________ mol”¤L£1”£
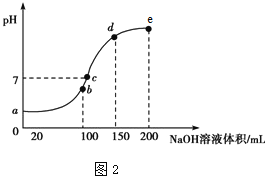
(4)ŹŅĪĀŹ±£¬Ļņ100 mL 0.1 mol”¤L£1 NH4HSO4ČÜŅŗÖŠµĪ¼Ó 0.1 mol”¤L£1 NaOHČÜŅŗ£¬ČÜŅŗpHÓėNaOHČÜŅŗĢå»żµÄ¹ŲĻµĒśĻßČēÉĻĶ¼2ĖłŹ¾”£
ŹŌ·ÖĪöĶ¼ÖŠ a”¢b”¢c”¢dĖÄøöµć£¬Ė®µÄµēĄė³Ģ¶Č×ī“óµÄŹĒ___________µć£¬ŌŚbµć£¬ČÜŅŗÖŠø÷Ąė×ÓÅضČÓɓ󵽊”µÄÅÅĮŠĖ³ŠņŹĒ_____________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æN2O5ŌŚŅ»¶ØĪĀ¶ČĻĀæÉ·¢Éś·“Ó¦£ŗ2N2O5(g) ![]() 4NO2(g)+O2(g) ”÷H>0”£T1ĪĀ¶ČŹ±£¬ĻņĆܱÕČŻĘ÷ÖŠĶØČėN2O5ĘųĢ壬²æ·ÖŹµŃ鏿¾Ż¼ūĻĀ±ķ£ŗ
4NO2(g)+O2(g) ”÷H>0”£T1ĪĀ¶ČŹ±£¬ĻņĆܱÕČŻĘ÷ÖŠĶØČėN2O5ĘųĢ壬²æ·ÖŹµŃ鏿¾Ż¼ūĻĀ±ķ£ŗ
Ź±¼ä/s | 0 | 500 | 1000 | 1500 |
c(N2O5) / (mol/L) | 5.00 | 3.52 | 2.50 | 2.50 |
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
A. 500 sÄŚ NO2µÄÉś³ÉĖŁĀŹĪŖ 2. 96”Į10-3mol/(L”¤s)
B. T1ĪĀ¶ČĻĀøĆ·“Ó¦Ę½ŗāŹ±N2O5µÄ×Ŗ»ÆĀŹĪŖ29.6%
C. Ę½ŗāŗó£¬ĘäĖūĢõ¼ž²»±ä£¬½«ČŻĘ÷Ģå»ż±äŌĄ“µÄ1/2,Ōņc(N2O5)<5.00mol/L
D. T1”¢T2ĪĀ¶ČĻĀµÄĘ½ŗā³£Źż·Ö±šĪŖK1”¢K2£¬ČōT1>T2£¬ŌņK1>K2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æX”¢Y”¢Z”¢M”¢WĪŖĪåÖÖ¶ĢÖÜĘŚŌŖĖŲ”£X”¢Y”¢ZŹĒŌ×ÓŠņŹżŅĄ“ĪµŻŌöµÄĶ¬ÖÜĘŚŌŖĖŲ£¬ĒŅ×īĶā²ćµē×ÓŹżÖ®ŗĶĪŖ15£¬XÓėZæÉŠĪ³ÉXZ2·Ö×Ó£»YÓėMŠĪ³ÉµÄĘųĢ¬»ÆŗĻĪļŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ0.76 g/L£»WµÄÖŹ×ÓŹżŹĒX”¢Y”¢Z”¢MĖÄÖÖŌŖĖŲÖŹ×ÓŹżÖ®ŗĶµÄ1/2”£ĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ
A. Ō×Ó°ė¾¶£ŗW£¾X£¾Y£¾Z£¾M
B. XZ2ĪŖÖ±ĻߊĪµÄ¹²¼Ū»ÆŗĻĪļ
C. X”¢Y”¢Z ·Ö±šÓėMŌŖĖŲŠĪ³ÉµÄ×ī¼ņµ„»ÆŗĻĪļµÄ·ŠµćŅĄ“ĪÉżøß
D. ÓÉX”¢Y”¢Z”¢MĖÄÖÖŌŖĖŲŠĪ³ÉµÄ»ÆŗĻĪļŅ»¶Øŗ¬ÓŠĄė×Ó¼üŗĶ¹²¼Ū¼ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æFe3+”¢SO42-”¢Al3+ŗĶXĖÄÖÖĄė×ÓŅŌĪļÖŹµÄĮæÖ®±Č2:4:1:1“óĮæ¹²“ęÓŚĶ¬Ņ»ČÜŅŗÖŠ£¬XæÉÄÜŹĒ
A£®Na+ B£®OHØC C£®CO32- D£®ClØC
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠ¹ŲÓŚÅØĻõĖįŗĶÅØĮņĖįµÄŠšŹö£¬ÕżČ·µÄŹĒ£Ø £©
A.³£ĪĀĻĀ¶¼ÓĆĶČŻĘ÷Öü“ę
B.Ā¶ÖĆŌŚæÕĘųÖŠ£¬ČŻĘ÷ÄŚĖįŅŗµÄÖŹĮ涼¼õĒį
C.³£ĪĀĻĀ¶¼ÄÜÓėĶ½Ļæģ·“Ó¦
D.Ā¶ÖĆŌŚæÕĘųÖŠ£¬ČŻĘ÷ÄŚĖįŅŗµÄÅØ¶Č¶¼½µµĶ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com