

��ش��������⣺
(1)������ϩ��һ����Ҫ��ҵ��;��___________________________________________��
(2)ʵ������ȡ��ϩ�Ļ�ѧ����ʽΪ__________________________________��
(3)D�Ľṹ��ʽΪ__________________________________��
(4)I(C6H10O4)�ж���ͬ���칹�壬���з���HOOC��COOC4H9��ͬ���칹�干��______________�֡�
(5)д������ת���Ļ�ѧ����ʽ��ָ��ָ����Ӧ�ķ�Ӧ���͡�
E![]() F��__________________________________��
F��__________________________________��
G+H![]() I��___________________________����Ӧ���ͣ�_________________��
I��___________________________����Ӧ���ͣ�_________________��
(1)��ȡ�Ҵ����������ϡ��ϳ���ά���л��ܼ���
(2)CH3CH2OH![]() CH2=CH2��+H2O
CH2=CH2��+H2O
(3)![]()
(4)4
(5)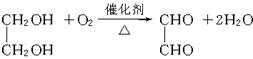
2CH3CH2OH+HOOCCOOH![]() CH3CH2OOCCOOCH2CH3+2H2O
CH3CH2OOCCOOCH2CH3+2H2O
������Ӧ
������(1)�������Һ����������֪ʶ�����ѵó���ϩ��һ����Ҫ��ҵ��;.
(4)����HOOC��COOC4H9��ͬ���칹�������ȡ���ڶ���(��C4H9)�����������ڡ�C4H9����4�֣����Է���������ͬ���칹����4��.
(5)�Ҷ����к������ǻ�����������Ӧʱ���ʵ���֮��Ϊ1��1.����HOOC��COOH�к��������Ȼ��������Ҷ�����C2H5OH����������Ӧʱ���ʵ���֮��Ϊ1��2����Ϊ���淴Ӧ.



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��ش��������⣺
��1��������ϩ��һ����Ҫ��ҵ��;��_____________________��
��2��ʵ������ȡ��ϩ�Ļ�ѧ����ʽΪ________________________��
��3��D�Ľṹ��ʽΪ__________________��
��4��I��C6H10O4���ж���ͬ���칹�壬���з���HOOC��COOC4H9��ͬ���칹�干��___________�֡�
��5��д������ת���Ļ�ѧ����ʽ��ָ��ָ����Ӧ�ķ�Ӧ���͡�
E![]() F��__________________________��
F��__________________________��
G+H![]() I��___________________����Ӧ���ͣ�_________________________��
I��___________________����Ӧ���ͣ�_________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com