
�����£���0.1 mol��L��1��������Һ����μ������ʵ���Ũ����ͬ������������Һ�����ɳ����������������������Һ�������ϵ��ͼ��ʾ��a��b��c��d�ֱ��ʾʵ�鲻ͬʱ�̵���Һ�������й�˵������ȷ����
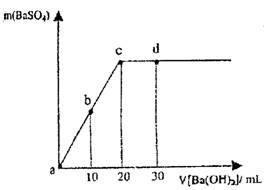 A��cʱ��������Һ�����Ϊ30 mL
A��cʱ��������Һ�����Ϊ30 mL
B��bʱ����Һ��SO42����Ũ��ԼΪ0.5 mol��L��1
C��dʱ����Һ��OH-��Ũ��Ϊ0.04mol/L
D����Һ�ĵ���������c<d=b<a
C
Ҫ����ͼ�����㡢�յ�ĺ��塣�������������������кͷ�Ӧ��C��ǡ�÷�Ӧ��ȫ����Ϊ����Ũ����ͬ�����������ȣ�A�����bʱ��SO42������һ�룬�����Ϊԭ����1.5����SO42����Ũ��ԼΪ0.1 /1.5=0.0667mol��L��1��B�����dʱ��Ba(OH)2������c��OH-��=��0.1��10��2��/50=0.04mol/L,C����ȷ��b��d�����������ʵ�����ȣ����������������Ũ��С��������������������c<d<b<a��D�����ѡC��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ᡢ��ζ��ǵ���ʣ���ˮ�ж��ܵ�������ӣ��������л����
�ᡢ��ζ��ǵ���ʣ���ˮ�ж��ܵ�������ӣ��������л�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������HCl ��NaOH ��CH3COOH ��NH3?H2O ��CH3COONa ��NH4Cl
���л������HCl ��NaOH ��CH3COOH ��NH3?H2O ��CH3COONa ��NH4Cl�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����£���100mL 0.01mol?L-1 HA��Һ����μ���0.02mol?L-1 MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯�������Һ����仯���Բ��ƣ�������˵���У���ȷ���ǣ�������
�����£���100mL 0.01mol?L-1 HA��Һ����μ���0.02mol?L-1 MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯�������Һ����仯���Բ��ƣ�������˵���У���ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2012�������ǰ��Ϣ����ѧ���� ���ͣ�021
|
�����йص������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���� | |
A�� |
�����£���0.1 mol��L��1��NH4Cl��Һ��0.05 mol��L��1��NaOH��Һ�������ϣ�c(Cl��)��c(Na+)��c(NH4+)��c(OH��)��c(H+) |
B�� |
�����£���CH3COOH��Һ�м���������NaOH���õ�pH��4�Ļ����Һ��c(Na+)��c(CH3COO��)��c(H+)��c(OH��) |
C�� |
Ũ�Ⱦ�Ϊ0.1 mol��L��1��Na2CO3��Һ��NaHCO3��Һ�������ϣ�c(Na+)��c(H+)��2c(CO32��)��c(OH��)��c(HCO3��) |
D�� |
�����£�pH��3��һԪ��HX��Һ��pH��11��һԪ��MOH��Һ�������ϣ�c(M+)��c(X��)��c(H+)��c(OH��) |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com