
(15��)
X��Y��W��M��N����Ԫ�طֱ�λ�����ڱ����������ڵ����ڣ���ԭ������������X��Y���⻯�ﶼ��ͬ��Ԫ���⻯��ķе�ߣ�����ͬ������ȴ������ߵġ�W��ͬ����Ԫ�������Ӱ뾶��С��Ԫ�ء�Mԭ�ӵ������ܲ����������˶�״̬��ͬ�ĵ��ӡ�N��һ�֡����ǽ��������㷺Ӧ���ں��졢���µȹ�ҵ������ش��������⣺
��X��Y����Ԫ�ص�Ԫ�ط����ǣ� �� ��X��Y�����γ�һ�ֹ��ۻ������������Ԫ���������������ﵽ8��������ӵĿռ乹���ǣ� ������ԭ�ӵ��ӻ���ʽ�ǣ� ��
��X���⻯��������ˮ����ԭ���ǣ� ��
��N�ĵ����Ų�ʽ�ǣ� ��
��X��Y��Ԫ�صĵ�һ�����ܴ�С��ϵ�� С�� (��Ԫ�ط���)��
��M��Y�γɵĻ�����ľ����������ڣ� ���侧����ͼ��ʾ������M���ӵ���λ���ǣ� ��

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 2a+32b |
| a+b |
| 2a+32b |
| a+b |
| 32(a+b) |
| 16a+b |
| 32(a+b) |
| 16a+b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
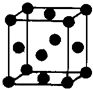 ��2012?�人ģ�⣩[��ѧһѡ�� 3�����ʽṹ������]��U��V��W��X��Y��Z ����ǰ������Ԫ�أ�ԭ���������������������Ϣ���±���
��2012?�人ģ�⣩[��ѧһѡ�� 3�����ʽṹ������]��U��V��W��X��Y��Z ����ǰ������Ԫ�أ�ԭ���������������������Ϣ���±���| Ԫ�ر�� | �����Ϣ�� |
| U | ���������������������ֱ�����ԭ��������� |
| V | ��̬ʱ�����ӷֲ��������ܼ��ϣ��Ҹ��ܼ��е�������� |
| W | ��̬ʱ��2p ������ڰ����״̬ |
| X | ��WԪ�ش���ͬһ���ڣ���X�ĵ�һ������С��W�ĵ�һ���� |
| Y | �ǵ�������Ԫ����δ�ɶԵ���������Ԫ�� |
| Z | Z ��һ�ֺ��ص�������Ϊ65��������Ϊ 36 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(15��)��X��Y��Z����Ԫ�أ�X���л��������бغ��е�Ԫ�أ�Y�ǵؿ��ﺬ����ߵ�Ԫ�أ�Z�����������Ԫ�أ�X��Y�ܽ�ϳ����ֻ�����A��B��A����ȼ�գ�B����ȼ��Ҳ����֧��ȼ�գ�X��Z��ϵĻ�����C�����������X��Y��Z����Ԫ����ɵĻ�����D������ʵ���Ҽ��ȵ�ȼ�ϡ�
��1�����ж�X��Y��Z����ʲôԪ�أ������ƣ���X ��Y ��Z ��
��2�����ж�A��B��C��D����ʲô���ʣ��ѧʽ����
A ��B ��C ��D ��
��3��д��������A��Fe2O3��Ӧ�Ļ�ѧ����ʽ ��
��4����֪������8g C��O2����ȫȼ�շų�444.8kJ����������д��������C��ȫȼ�յ��Ȼ�ѧ����ʽ�� ��
��5�����û�����D��Na��Ӧ��ȡ���ռ�1g H2����ҪD������ g����д��������D����Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ���Ƹ���ѧ��һ�ڶ�ѧ����ĩ���Ի�ѧ���� ���ͣ������
(15��)��X��Y��Z����Ԫ�أ�X���л��������бغ��е�Ԫ�أ�Y�ǵؿ��ﺬ����ߵ�Ԫ�أ�Z�����������Ԫ�أ�X��Y�ܽ�ϳ����ֻ�����A��B��A����ȼ�գ�B����ȼ��Ҳ����֧��ȼ�գ�X��Z��ϵĻ�����C�����������X��Y��Z����Ԫ����ɵĻ�����D������ʵ���Ҽ��ȵ�ȼ�ϡ�
��1�����ж�X��Y��Z����ʲôԪ�أ������ƣ���X ��Y ��Z ��
��2�����ж�A��B��C��D����ʲô���ʣ��ѧʽ����
A ��B ��C ��D ��
��3��д��������A��Fe2O3��Ӧ�Ļ�ѧ����ʽ ��
��4����֪������8g C��O2����ȫȼ�շų�444.8kJ����������д��������C��ȫȼ�յ��Ȼ�ѧ����ʽ�� ��
��5�����û�����D��Na��Ӧ��ȡ���ռ�1g H2����ҪD������ g����д��������D�� ��Ӧ�Ļ�ѧ����ʽ�� ��
��Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ��һ�ڶ�ѧ����ĩ���Ի�ѧ���� ���ͣ������
(15��)��X��Y��Z����Ԫ�أ�X���л��������бغ��е�Ԫ�أ�Y�ǵؿ��ﺬ����ߵ�Ԫ�أ�Z�����������Ԫ�أ�X��Y�ܽ�ϳ����ֻ�����A��B��A����ȼ�գ�B����ȼ��Ҳ����֧��ȼ�գ�X��Z��ϵĻ�����C�����������X��Y��Z����Ԫ����ɵĻ�����D������ʵ���Ҽ��ȵ�ȼ�ϡ�
��1�����ж�X��Y��Z����ʲôԪ�أ������ƣ���X ��Y ��Z ��
��2�����ж�A��B��C��D����ʲô���ʣ��ѧʽ����
A ��B ��C ��D ��
��3��д��������A��Fe2O3��Ӧ�Ļ�ѧ����ʽ ��
��4����֪������8g C��O2����ȫȼ�շų�444.8kJ����������д��������C��ȫȼ�յ��Ȼ�ѧ����ʽ�� ��
��5�����û�����D��Na��Ӧ��ȡ���ռ�1g H2����ҪD������
g����д��������D�� ��Ӧ�Ļ�ѧ����ʽ��
��
��Ӧ�Ļ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com