

 ��
������ ���������Ҫ�ɷ�ΪMg2B2O5•H2O��Fe3O4����������Fe2O3��FeO��CaO��Al2O3��SiO2�ȣ�Ϊԭ���Ʊ����ᣨH3BO3���������̿�֪���������ܽ⣬Fe3O4��SiO2���ܣ�CaOת��Ϊ����ˮ��CaSO4��Fe3O4���д��ԣ����Բ��������������룬����1�ijɷ�ΪSiO2��CaSO4��
���������ӡ����ȼ�H2O2��Һ����Fe 2+ת��ΪFe 3+��������Һ��pHԼΪ5��ʹFe3+��Al3+��ת��Ϊ������������ΪAl��OH��3��Fe��OH��3��Ȼ������Ũ������ȴ�ᾧ�����˷����H3BO3��
��1��Mg2B2O5•H2O�����ᷴӦ��������þ�����
��2���������������д��ԣ�Fe3O4��SiO2������ϡ���ᣬCaSO4�������
��3��NaBH4Ϊ���ӻ���������Ӽ������ۼ��������ʽΪ ��
��
��4��˫��ˮ���������ԣ��ܽ���ԭ�����������������������ӣ�������Һ��pH��5����ʱFe3+��Al3+��ת��Ϊ������
��5������������Ҫ����Ϊ�����Ե�����þ���壮
��� �⣺���������Ҫ�ɷ�ΪMg2B2O5•H2O��Fe3O4����������Fe2O3��FeO��CaO��Al2O3��SiO2�ȣ�Ϊԭ���Ʊ����ᣨH3BO3���������̿�֪���������ܽ⣬Fe3O4��SiO2���ܣ�CaOת��Ϊ����ˮ��CaSO4��Fe3O4���д��ԣ����Բ��������������룬����1�ijɷ�ΪSiO2��CaSO4��
���������ӡ����ȼ�H2O2��Һ����Fe 2+ת��ΪFe 3+��������Һ��pHԼΪ5��ʹFe3+��Al3+��ת��Ϊ������������ΪAl��OH��3��Fe��OH��3��Ȼ������Ũ������ȴ�ᾧ�����˷����H3BO3��
��1��Mg2B2O5•H2O�����ᷴӦ��������þ�����ᣬ��Ӧ����ʽΪMg2B2O5•H2O+2H2SO4�T2MgSO4+2H3BO3���ʴ�Ϊ��Mg2B2O5•H2O+2H2SO4�T2MgSO4+2H3BO3��
��2������Fe3O4�Ĵ��ԣ��ɽ���ӡ��������з��룮���������л�ʣ���������SiO2��CaSO4���ʴ�Ϊ��Fe3O4��SiO2��CaSO4��
��3��NaBH4Ϊ���ӻ���������Ӽ������ۼ��������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4�����������ӡ����ȼ�H2O2��Һ�������ǽ�������������Ϊ�����ӣ�Ȼ���ڵ�����Һ��pHԼΪ5��Ŀ����ʹ�����ӡ��������γ����������������ȥ��
�ʴ�Ϊ����Fe2+����ΪFe3+��ʹAl3+��Fe3+�γ����������������ȥ��
��5�����Ũ���ᾧʱ����þ���ˮ�Ծ��������������ᡱ�е���Ҫ��������ˮ����þ���ʴ�Ϊ����ˮ����þ��
���� ���⿼�����ʷ�����ᴿ���ۺ�Ӧ�ã�Ϊ��Ƶ���㣬�漰����ʵ���������ѧ���������ԭ��Ӧ��֪ʶ�㣬����ʵ�����̼������ķ�Ӧ�����������ᴿ����Ϊ���Ĺؼ������ط�����ʵ���������ۺϿ��飬��Ŀ�ѶȲ���


 ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuO����ϡH2SO4 | B�� | NaOH��Һ��HNO3��Һ��Ӧ | ||
| C�� | KOH��Һ��CH3COOH��Һ��Ӧ | D�� | Ba��OH��2 ��Һ��H2SO4��Һ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HClO4�� H2SO4 | B�� | CH3COOH��H2Se | C�� | C2H5OH��NaOH | D�� | H2O2��HNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 65.4% | B�� | 52.9% | C�� | 47.1% | D�� | 34.6% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4.6g�Ҵ����������Ʒ�Ӧ�ų�1.12L���� | |
| B�� | ���ñ�����ʹ���ˮ����ȡ�� | |
| C�� | 0.1mol�����к���Լ6.02��1023�����ۼ� | |
| D�� | ����ʽΪC4H9Cl���л��ﹲ��5�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �е㣺NH3��H2O��HF��HCl | B�� | ���ʵ��۵㣺ʯӢ��ʳ�Σ��⣾�� | ||
| C�� | ������ӣ�H+����������OH-��NH3��H2O | D�� | ���ӵ�ֱ����S2-��Cl-��K+��Ca2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
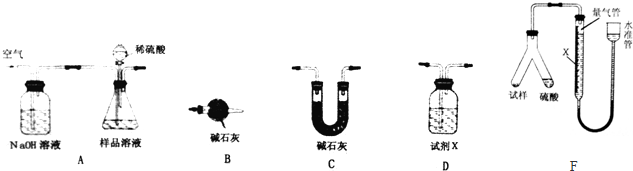
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ǽ����ȡ��������γɺϽ� | B�� | �Ͻ��ǻ���� | ||
| C�� | �γɺϽ�����۵�Ҫ��� | D�� | �γɺϽ����Ӳ��Ҫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������������Һ�����ô������IJ���ƿ | |
| B�� | ����Ũ���ᣬ���ô�����������ɫ�Լ�ƿ | |
| C�� | ���������ʱ�������Լ�ƿ�м���ú�ͽ��з�� | |
| D�� | ���������ʱ����������ƿ�����ò���ƿ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com