
ij�о���ѧϰС����CO��ԭFe2O3��ʵ���У��ô����������ɵĺ�ɫ��ĩX��������ΪX��һ����Fe�����¶Ȳ���ʱ������Fe3O4��Ҳ�ܱ�����������Ϊ��̽��X����ɣ����ǽ���������ʵ�顣
I�����Լ���
| ʵ���� | ʵ����� | ʵ������ |
| �� | ȡ������ɫ��ĩX�����Թ�1�У�ע��Ũ���ᣬ�� | ��ɫ��ĩ���ܽ⣬��Һ�ʻ���ɫ�����������ݲ��� |
| �� | ��ȡ������ɫ��ĩX�����Թ�2�У�ע����������ͭ��Һ�������� | �м�������ɫ�������������н϶��ɫ����δ�ܽ� |
������ʵ�������ƶϣ���ɫ��ĩX�ijɷ��� ��
II�������ⶨ
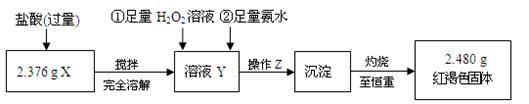
�� ����Z�������� ����ͼ19�������У������ճ���ʱ�����õ����� ������ĸ����
�� д����ҺY�еμ�H2O2��Һʱ������Ӧ�����ӷ���ʽ��
��
�� ����������ȣ������ڸ���������ȴ�����£���������ƽ����������Ϊb1 g���ٴμ��Ȳ���ȴ�����³���������Ϊb2 g����b1 �� b2 �� 0.3 g�����������Ӧ���еIJ�����
��
�� ��ͬѧ��Ϊ������������������H2O2���������費�䣬ֻҪ�ڿ����г�ַ����ԿɴﵽĿ�ġ����������ǣ��û�ѧ����ʽ��ʾ����
��
�� ͨ���������ݣ��ó�2.376 g��ɫ��ĩ�и��ɷֵ����ʵ���Ϊ ��
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| c��NO������10-4mol?L-1�� | 10.0 | 4.50 | 2.50 | 1.50 | 1.00 | 1.00 |
| c��NO������10-3mol?L-1�� | 3.60 | 3.05 | 2.85 | 2.75 | 2.70 | 2.70 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ʵ�������õ��ĵζ��ܡ�����ƿ����ʹ��ǰ����Ҫ��© | B�����ʵ��������60mL ��ϡ�������Һ������ʱӦѡ��100mL����ƿ | C������ƿ�к�����������ˮ���ᵼ���������Һ��Ũ��ƫС | D����ʽ�ζ���������ˮϴ�Ӻ�װ���Ũ�ȵ�ϡ���ᣬ���õ�NaOH��Һ��Ũ�Ƚ�ƫ�� | E��������Һʱ���������һ�ζ���ʱ���Ӷ������������ʵ����ƫ�� | F���к͵ζ�ʱ���������һ�ζ���ʱ���Ӷ������������ʵ����ƫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
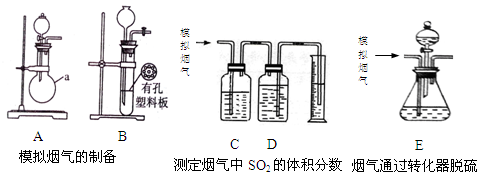
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com