
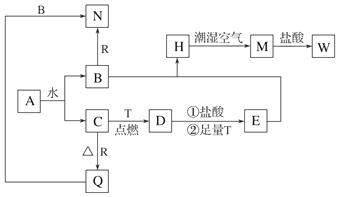
·ÖĪö AĪŖµ»ĘÉ«¹ĢĢ壬ĒŅAÄÜŗĶĖ®·“Ó¦£¬ŌņAŹĒNa2O2£¬AŗĶĖ®·“Ӧɜ³ÉNaOHŗĶO2£¬CŹĒĪŽÉ«ĪŽĪ¶µÄĘųĢ壬ŌņCŹĒO2”¢BŹĒNaOH£»DŹĒ¾ßÓŠ“ÅŠŌµÄŗŚÉ«¾§Ģ壬ŌņDŹĒFe3O4£¬FeŌŚŃõĘųÖŠČ¼ÉÕÉś³ÉĖÄŃõ»ÆČżĢś£¬ŌņTŹĒFe£¬RŗĶŃõĘų·“Ӧɜ³ÉŃõ»ÆĪļQ£¬QÄÜŗĶNaOHČÜŅŗ·“Ó¦£¬ŌņQŹĒAl2O3”¢RŹĒAl£¬NŹĒNaAlO2£¬HŹĒ°×É«³Įµķ£¬ĒŅŌŚ³±ŹŖæÕĘųÖŠŃøĖŁ±äĪŖ»ŅĀĢÉ«£¬×īÖÕ±äĪŖŗģŗÖÉ«¹ĢĢåM£¬ŌņHŹĒFe£ØOH£©2”¢MŹĒFe£ØOH£©3£¬Fe3O4ŗĶHCl”¢Fe·“Ó¦ŗóµĆµ½E£¬EĪŖFeCl2£¬Fe£ØOH£©3ŗĶHCl·“Ӧɜ³ÉW£¬ŌņWŹĒFeCl3£¬ŅŌ“Ė½ā“šøĆĢā£®
½ā“š ½ā£ŗ£Ø1£©ÓÉŅŌÉĻ·ÖĪöæÉÖŖDĪŖFe3O4£¬QĪŖAl2O3£¬¹Ź“š°øĪŖ£ŗFe3O4£»Al2O3£»
£Ø2£©AŹĒNa2O2£¬AŗĶĖ®·“Ӧɜ³ÉNaOHŗĶO2£¬·½³ĢŹ½ĪŖ2Na2O2+2H2O=4NaOH+O2”ü£¬¹Ź“š°øĪŖ£ŗ2Na2O2+2H2O=4NaOH+O2”ü£»
£Ø3£©EĪŖFeCl2£¬WŹĒFeCl3£¬Č·¶ØEČÜŅŗÖŠŹĒ·ńŗ¬ÓŠWĪļÖŹ£¬æɼÓČėKSCN¼ģŃ飬¹Ź“š°øĪŖ£ŗb£»
£Ø4£©Fe£ØOH£©2²»ĪČ¶Ø£¬Ņ×±»Ńõ»ÆÉś³ÉĒāŃõ»ÆĢś£¬æɹŪ²ģµ½µÄĻÖĻóĪŖÉś³É°×É«³Įµķ£¬³ĮµķŃøĖŁ±ä³É»ŅĀĢÉ«£¬×īŗó±ä³ÉŗģŗÖÉ«£¬
¹Ź“š°øĪŖ£ŗÉś³É°×É«³Įµķ£¬³ĮµķŃøĖŁ±ä³É»ŅĀĢÉ«£¬×īŗó±ä³ÉŗģŗÖÉ«£»
£Ø5£©ĀĮŗĶNaOHČÜŅŗ·“Ӧɜ³ÉĘ«ĀĮĖįÄĘŗĶĒāĘų£¬Ąė×Ó·“Ó¦·½³ĢŹ½ĪŖ2Al+2OH-+2H2OØT2AlO2-+3H2”ü£¬WŹĒFeCl3£¬TŹĒFe£¬¶žÕß·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖFe+2Fe3+=3Fe2+£¬
¹Ź“š°øĪŖ£ŗ2Al+2OH-+2H2O=2AlO2-+3H2”ü£»Fe+2Fe3+=3Fe2+£®
µćĘĄ ±¾ĢāŅŌNa”¢Al”¢Fe¼°Ęä»ÆŗĻĪļĪŖŌŲĢåæ¼²éĮĖ½šŹōŌŖĖŲ¼°Ęä»ÆŗĻĪļµÄĶʶĻ£¬øł¾ŻHµÄŃÕÉ«±ä»Æ”¢AµÄŃÕÉ«¼°ŠŌÖŹ”¢DµÄŠŌÖŹĪŖĶ»ĘĘæŚ²ÉÓĆÕżÄę½įŗĻµÄ·½·Ø½ųŠŠĶʶĻ£¬ŹģĻ¤ĪļÖŹŠŌÖŹŹĒ½ā±¾Ģā¹Ų¼ü£¬ŌŁ½įŗĻĪļÖŹ¼äµÄ×Ŗ»ÆĄ“½ā“š£¬ĢāÄæÄѶČÖŠµČ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| BŌŖĖŲŌ×ÓµÄŗĖĶāpµē×ÓŹż±Čsµē×ÓŹżÉŁ1 |
| CŌ×ӵĵŚŅ»ÖĮµŚĖĵēĄėÄÜ·Ö±šŹĒ£ŗI1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| DŌ×ÓŗĖĶāĖłÓŠp¹ģµĄČ«Āś»ņ°ėĀś |
| EŌŖĖŲµÄÖ÷×åŠņŹżÓėÖÜĘŚŹżµÄĻą²ī4 |
| F ŹĒĒ°ĖÄÖÜĘŚŌ×Óµē×ÓÅŲ¼Ķ¼ÖŠµ„µē×ÓŹż×ī¶ąµÄŌŖĖŲ |
| GŌŚÖÜĘŚ±ķµÄµŚŹ®Ņ»ĮŠ |
 £®
£®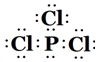 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 16gO2Õ¼ÓŠµÄĢå»żŌ¼ĪŖ11.2L | |
| B£® | 22.4LH2ŗ¬ÓŠ°¢·ü¼ÓµĀĀŽ³£ŹżøöĒā·Ö×Ó | |
| C£® | ŌŚ±ź×¼×“æöĻĀ£¬44.8LH2OµÄÖŹĮæŌ¼ĪŖ36g | |
| D£® | 11gCO2Óė±ź×¼×“æöĻĀ5.6LHClŗ¬ÓŠĻąĶ¬µÄ·Ö×ÓŹż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ź±¼ä/s | 0 | 20 | 40 | 60 | 80 | 100 |
| c£ØNO2£©/mol•L-1 | 0.00 | 0.12 | 0.20 | 0.26 | 0.30 | 0.30 |
| A£® | 20”«40sÄŚ£¬v£ØN2O4£©=0.004mol/£ØL•s£© | |
| B£® | ŌŚĻąĶ¬Ģõ¼žĻĀ£¬æŖŹ¼Ź±ČōĻņČŻĘ÷ÖŠ³äČėµÄŹĒ0.80 molNO2£¬“ļµ½Ę½ŗāŗóNO2µÄ×Ŗ»ÆĀŹĪŖ75% | |
| C£® | ·“Ó¦“ļĘ½ŗāŹ±£¬ĪüŹÕµÄČČĮæĪŖ15.9 kJ | |
| D£® | 100 sŹ±ŌŁĶØČė0.40 mol N2O4£¬“ļŠĀĘ½ŗāŹ±N2O4µÄ×Ŗ»ÆĀŹŌö“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
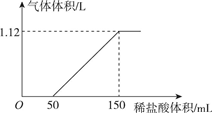 ĻņijĢ¼ĖįÄĘŗĶĢ¼ĖįĒāÄʵĻģŗĻČÜŅŗÖŠÖšµĪ¼ÓČė0.05mol/LµÄĻ”ŃĪĖį£¬¼ÓČėĻ”ŃĪĖįĢå»żÓė±ź×¼×“æöĻĀ²śÉśĘųĢåĢå»żµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®
ĻņijĢ¼ĖįÄĘŗĶĢ¼ĖįĒāÄʵĻģŗĻČÜŅŗÖŠÖšµĪ¼ÓČė0.05mol/LµÄĻ”ŃĪĖį£¬¼ÓČėĻ”ŃĪĖįĢå»żÓė±ź×¼×“æöĻĀ²śÉśĘųĢåĢå»żµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
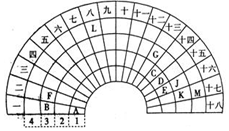 ŌŖĖŲÖÜĘŚ±ķµÄŠĪŹ½¶ąÖÖ¶ąŃł£¬ČēĶ¼ŹĒÉČŠĪŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£ØĒ°ĖÄÖÜĘŚµÄŌŖĖŲ£©£¬¶Ō±Č֊ѧ³£¼ūŌŖĖŲÖÜĘŚ±ķ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
ŌŖĖŲÖÜĘŚ±ķµÄŠĪŹ½¶ąÖÖ¶ąŃł£¬ČēĶ¼ŹĒÉČŠĪŌŖĖŲÖÜĘŚ±ķµÄŅ»²æ·Ö£ØĒ°ĖÄÖÜĘŚµÄŌŖĖŲ£©£¬¶Ō±Č֊ѧ³£¼ūŌŖĖŲÖÜĘŚ±ķ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ £®
£® £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
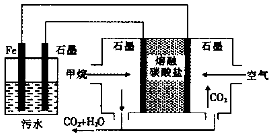
| A£® | Feµē¼«µÄµē¼«·“Ó¦Ź½ĪŖ£ŗFe-2e-ØTFe2+ | |
| B£® | ĶØČėæÕĘųµÄŹÆÄ«µē¼«µÄµē¼«·“Ó¦Ź½ĪŖO2+2CO2+4e-ØT2CO32- | |
| C£® | ĶØČė¼×ĶéµÄŹÆÄ«µē¼«µÄµē¼«·“Ó¦Ź½ĪŖ£ŗCH4+100H+-8e-ØTCO32-+7H2O | |
| D£® | ĪŖŌöĒæĪŪĖ®µÄµ¼µēÄÜĮ¦£¬æÉĻņĪŪĖ®ÖŠ¼ÓČėŹŹĮ湤ŅµÓĆŹ³ŃĪ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  ·ÖĄėøŹÓĶŗĶĖ® ·ÖĄėøŹÓĶŗĶĖ® | B£® | 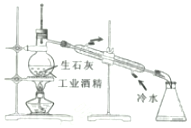 ÓĆ¹¤Ņµ¾Ę¾«ÖĘČ”ĪŽĖ®¾Ę¾« ÓĆ¹¤Ņµ¾Ę¾«ÖĘČ”ĪŽĖ®¾Ę¾« | ||
| C£® | 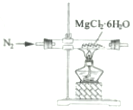 ÖĘČ”MgCl2¹ĢĢå ÖĘČ”MgCl2¹ĢĢå | D£® | 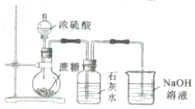 ¼ģ²āÕįĢĒÓėÅØĮņĖį·“Ó¦²śÉśµÄCO2 ¼ģ²āÕįĢĒÓėÅØĮņĖį·“Ó¦²śÉśµÄCO2 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com