
������(H3PO2)��һ�־�ϸ������Ʒ�����н�ǿ��ԭ�ԡ��ش��������⣺
(1)H3PO2��һ����ǿ�ᣬд������뷽��ʽ��_____________________________
________________________________________________________________________��
(2)H3PO2��NaH2PO2���ɽ���Һ�е�Ag����ԭΪ�����Ӷ������ڻ�ѧ������
��H3PO2�У�PԪ�صĻ��ϼ�Ϊ________��
������H3PO2���л�ѧ������Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ4��1������������Ϊ________(�ѧʽ)��
��NaH2PO2Ϊ________(����Ρ�����ʽ�Ρ�)������Һ��________(������ԡ������ԡ��������ԡ�)��
(3)H3PO2�Ĺ�ҵ�Ʒ��ǣ�������(P4)��Ba(OH)2��Һ��Ӧ����PH3�����Ba(H2PO2)2����������H2SO4��Ӧ��д��������Ba(OH)2��Һ��Ӧ�Ļ�ѧ����ʽ��____________________________________________��
(4)H3PO2Ҳ���õ��������Ʊ��������ҵ�������������ԭ����ͼ��ʾ(��Ĥ����Ĥ�ֱ�ֻ���������ӡ�������ͨ��)��
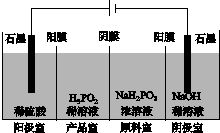
��д�������ĵ缫��Ӧʽ��_________________________________________��
�ڷ�����Ʒ�ҿɵõ�H3PO2��ԭ��_____________________________________
________________________________________________________________________��
�����ڲ��á����ҵ����������Ʊ�H3PO2���������ҵ����������������ҵ�ϡ������H3PO2ϡ��Һ���棬����ȥ���������Ʒ��֮�����Ĥ���Ӷ��ϲ������������Ʒ�ҡ���ȱ���Dz�Ʒ�л���________���ʣ������ʲ�����ԭ����________________________________��
(1)H3PO2H2PO ��H����(2)�٣�1����H3PO4�������Ρ�������
��H����(2)�٣�1����H3PO4�������Ρ�������
(3)2P4��3Ba(OH)2��6H2O��3Ba(H2PO2)2��2PH3��
(4)��2H2O��4e��===O2����4H��
�������ҵ�H��������Ĥ��ɢ����Ʒ�ң�ԭ���ҵ�H2PO ������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2����PO
������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2����PO ��H2PO
��H2PO ��H3PO2������
��H3PO2������
[����] (1)H3PO2ΪһԪ���ᣬ����뷽��ʽΪH3PO2 H����H2PO
H����H2PO ��(2)�ɻ��ϼ۴�����Ϊ0��ȷ��PΪ��1�ۣ��ڸ���������Ϣд����ѧ����ʽΪ4Ag����H3PO2��2H2O===4Ag��H3PO4��4H��������������ΪH3PO4����NaH2PO2Ϊǿ�������Σ���Һ�������ԡ�(3)����������Ϣ�ͷ�Ӧǰ��Ԫ�ػ��ϼ۱仯д����ѧ����ʽΪ2P4��3Ba(OH)2��6H2O===2PH3����3Ba(H2PO2)2��(4)��������ˮ�������OH���ŵ磬�䷴ӦʽΪ2H2O��4e��===O2����4H�������������е�H��������Ĥ�����Ʒ�ң�ԭ���ҵ�H2PO
��(2)�ɻ��ϼ۴�����Ϊ0��ȷ��PΪ��1�ۣ��ڸ���������Ϣд����ѧ����ʽΪ4Ag����H3PO2��2H2O===4Ag��H3PO4��4H��������������ΪH3PO4����NaH2PO2Ϊǿ�������Σ���Һ�������ԡ�(3)����������Ϣ�ͷ�Ӧǰ��Ԫ�ػ��ϼ۱仯д����ѧ����ʽΪ2P4��3Ba(OH)2��6H2O===2PH3����3Ba(H2PO2)2��(4)��������ˮ�������OH���ŵ磬�䷴ӦʽΪ2H2O��4e��===O2����4H�������������е�H��������Ĥ�����Ʒ�ң�ԭ���ҵ�H2PO ������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2�����������ڿ����в���H2PO
������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2�����������ڿ����в���H2PO ��H3PO2ʧ���ӷ���������Ӧ�������������л���PO
��H3PO2ʧ���ӷ���������Ӧ�������������л���PO ��
��


 �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ��䷴Ӧԭ��Ϊ
N2(g)��3H2(g) 2NH3(g)����H����92.4 kJ��mol��1��
2NH3(g)����H����92.4 kJ��mol��1��
һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�
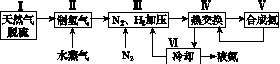
(1)��Ȼ���е�H2S���ʳ��ð�ˮ���գ�����ΪNH4HS��һ����������NH4HS��Һ��ͨ��������õ�������ʹ����Һ������д��������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(2)���������������ԭ�����£�
��CH4(g)��H2O(g)  CO(g)��3H2(g)
CO(g)��3H2(g)
��H����206.4 kJ��mol��1
��CO(g)��H2O(g)  CO2(g)��H2(g)
CO2(g)��H2(g)
��H����41.2 kJ��mol��1
���ڷ�Ӧ�٣�һ���������ƽ����ϵ��H2�İٷֺ��������ܼӿ췴Ӧ���ʵĴ�ʩ��____________��
a�������¶ȡ�b������ˮ����Ũ�ȡ�c�����������d������ѹǿ
���÷�Ӧ�ڣ���CO��һ��ת���������H2�IJ�������1 mol CO��H2�Ļ������(CO���������Ϊ20%)��H2O��Ӧ���õ�1.18 mol CO��CO2��H2�Ļ�����壬��CO��ת����Ϊ____________��
(3)ͼ(a)��ʾ500 �桢60.0 MPa�����£�ԭ����Ͷ�ϱ���ƽ��ʱNH3��������Ĺ�ϵ������ͼ��a�����ݼ���N2��ƽ�����������____________��
(4)�����¶ȶԺϳɰ���Ӧ��Ӱ�죬��ͼ(b)����ϵ�У�����һ�������µ��ܱ������ڣ���ͨ��ԭ������ʼ�����¶Ȳ������ߣ�NH3���ʵ����仯������ʾ��ͼ��
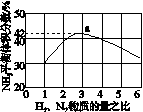 ��
��
(a)������������������(b)
(5)��������ͼ�У�ʹ�ϳɰ��ų��������õ�������õ���Ҫ������(�����)________����������������ߺϳɰ�ԭ����ת���ʵķ�����________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��״̬�£���̬���ӶϿ�1 mol��ѧ�����ʱ��Ϊ���ʡ���֪H��H��H��O��O===O���ļ��ʦ�H�ֱ�Ϊ436 kJ��mol��1��463 kJ��mol��1��495 kJ��mol��1�������Ȼ�ѧ����ʽ��ȷ����(����)
A��H2O(g)===H2��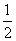 O2(g)����H����485 kJ��mol��1
O2(g)����H����485 kJ��mol��1
B��H2O(g)===H2(g)��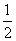 O2(g)����H����485 kJ��mol��1
O2(g)����H����485 kJ��mol��1
C��2H2(g)��O2(g)===2H2O(g)��
��H����485 kJ��mol��1
D��2H2(g)��O2(g)===2H2O(g)��
��H����485 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̵�ص����С�������������dz��õ�һ�ε�ء��õ�ط�Ӧԭ����ͼ��ʾ�����е����LiClO4�����ڻ���л��ܼ��У�Li��ͨ�������Ǩ����MnO2�����У�����LiMnO2�� �ش��������⣺
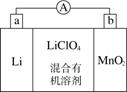
(1)���·�ĵ�����������________������________����(����ĸ)
(2)���������ӦʽΪ__________________________��
(3)�Ƿ����ˮ�������еĻ���л��ܼ���________(��ǡ���)��ԭ����________________________________________________��
(4)MnO2����KOH��KClO3�ڸ����·�Ӧ������K2MnO4����Ӧ�Ļ�ѧ����ʽΪ____________________________________��K2MnO4��������Һ���绯������KMnO2��MnO2�����ʵ���֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ⱦ�����Чȥ������Դ�ij�������ǻ�ѧ�츣�������Ҫ�о����⡣ij�о�С���������̿�(��Ҫ�ɷ�ΪMnO2��������������������ͭ�����Ƚ���������)���������ͨ�����¼����̼��ѳ�ȼúβ���е�SO2�����Ƶõ�ز���MnO2(��Ӧ������ʡ��)��
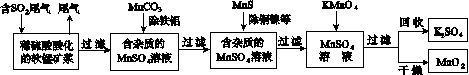
��ش��������⣺
(1)������������ʵ����____(ѡ��������ĸ���)��
A����������ۺ�����
B����ɫ��Ⱦ�ļ���
C������ļ���
(2)��MnCO3�ܳ�ȥ��Һ�е�Al3����Fe3������ԭ����________________________________��
(3)��֪��25 �桢101 kPaʱ��
Mn(s)��O2(g)===MnO2(s)����H����520 kJ/mol
S(s)��O2(g)===SO2(g)����H����297 kJ/mol
Mn(s)��S(s)��2O2(g)===MnSO4(s)��
��H����1065 kJ/mol
SO2��MnO2��Ӧ������ˮMnSO4���Ȼ�����ʽ��____________________________________��
(4)MnO2�����������������ϡ��ö��Ե缫���MnSO4��Һ���Ƶ�MnO2���������ĵ缫��Ӧʽ��________________________________��
(5)MnO2�Ǽ���п�̵�ص��������ϡ�����п�̵�طŵ�ʱ�������ĵ缫��Ӧʽ��________________��
(6)�����ѳ���SO2ֻ�����̿��е�MnO2��Ӧ������ͼʾ���̣���a m3(��״��)��SO2���������Ϊb%��β��ͨ�����SO2���ѳ���Ϊ89.6%�����յõ�MnO2������Ϊc kg�����ȥ��������ͭ����������ʱ�����������Ԫ���൱��MnO2________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��D��E��FΪ������Ԫ�أ��ǽ���Ԫ��A����������������������ͬ��B����������������������������2����B��D�г��ȼ������������ۻ�����BD2��E����D2��������ͬ�ĵ�������A��F��ȼ�գ���������ˮ�õ�һ��ǿ�ᡣ�ش��������⣺
(1)A�����ڱ��е�λ����________��д��һ�ֹ�ҵ�Ʊ�����F�����ӷ���ʽ��__________________________��
(2)B��D��E��ɵ�һ�����У�E����������Ϊ43%��������Ϊ__________����ˮ��Һ��F���ʷ�Ӧ�Ļ�ѧ����ʽΪ____________________________________________���ڲ����м�������KI����Ӧ�����CCl4�����л�����______ɫ��
(3)����ЩԪ����ɵ����ʣ�����ɺͽṹ��Ϣ���±���
| ���� | ��ɺͽṹ��Ϣ |
| a | ������A�Ķ�Ԫ���ӻ����� |
| b | �����зǼ��Թ��ۼ��Ķ�Ԫ���ӻ������ԭ����֮��Ϊ1��1 |
| c | ����ѧ���ΪBDF2 |
| d | ��ֻ����һ�������������ҿɵ���ĵ��ʾ��� |
a�Ļ�ѧʽΪ________��b�Ļ�ѧʽΪ______________��c�ĵ���ʽΪ________��d�ľ���������________��
(4)��A��B��DԪ����ɵ����ֶ�Ԫ�������γ�һ������Դ���ʡ�һ�ֻ��������ͨ��________�����ɾ��п�ǻ�Ĺ��壻��һ�ֻ�����(��������Ҫ�ɷ�)���ӽ���ÿ�ǻ������ӵĿռ�ṹΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Ҫ������Դ��������Ϊ�������õĹؼ��������ǵ�ǰ��ע���ȵ�֮һ��
(1)���������ȼ�ϣ���ȼ�ղ���Ϊ________��
(2)NaBH4��һ����Ҫ�Ĵ������壬����ˮ��Ӧ�õ�NaBO2���ҷ�Ӧǰ��B�Ļ��ϼ۲��䣬�÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________________����Ӧ����1 mol NaBH4ʱת�Ƶĵ�����ĿΪ________��
 (3)����ɽ����л�������û�����ͱ�֮��Ŀ��淴Ӧ��ʵ������ͼ��⣺
(3)����ɽ����л�������û�����ͱ�֮��Ŀ��淴Ӧ��ʵ������ͼ��⣺
 (g)
(g)  (g)��3H2(g)��
(g)��3H2(g)��
��ij�¶��£�������ܱ������м��뻷���飬����ʼŨ��Ϊa mol��L��1��ƽ��ʱ����Ũ��Ϊb mol��L��1���÷�Ӧ��ƽ�ⳣ��K��________��
(4)һ�������£���ͼ��ʾװ�ÿ�ʵ���л���ĵ绯ѧ����(���������л���)��
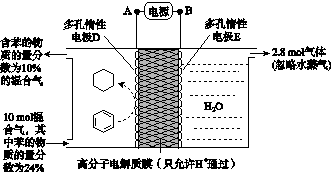
�ٵ����е����ƶ�����Ϊ________��(��A��D��ʾ)
������Ŀ�����ĵ缫��ӦʽΪ__________________��
�۸ô���װ�õĵ���Ч�ʦǣ�____________________��(�ǣ�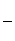 ��100%������������С�����1λ)
��100%������������С�����1λ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������������;�Ĺ�ϵ����ȷ���� ��
A���������ƣ������� ��B���ռ����θ������һ��ҩ��
C��С�մ��ͷ���Ҫ�ɷ� D����������ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����л������������ȷ����(����)
A����Ũ��ˮ��Ȼ����ˣ��ɳ�ȥ���л��е�������ϩ
B��������ˮ�����ۻ�Ͽ��Ƴ��屽
C�������ᡢ���۾����ڸ߷��ӻ�����
D����֪�ױ������ϵĶ���ȡ������6�֣���ױ������ϵ�����ȡ����Ҳ��6��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com