
£ØĆææÕ2·Ö£¬¹²16·Ö£©ÓŠX”¢Y”¢ZČżÖÖµ„ÖŹŗĶ¼×”¢ŅŅ”¢±ūČżÖÖ³£¼ūµÄ»ÆŗĻĪļ£¬ĖüĆĒÓŠČēĻĀĶ¼¼°ŠšŹöĖłŹ¾µÄ¹ŲĻµ£ŗ

£Ø1£©.X”¢Y”¢Z¶¼ŹĒ¶ĢÖÜĘŚŌŖĖŲµÄµ„ÖŹ£¬XŌŖĖŲŌ×ÓµÄ×īĶā²ćµē×ÓŹżŹĒĘä“ĪĶā²ćµē×ÓŹżµÄ2±¶£»YŌŖĖŲÓŠĮ½ÖÖ³£¼ūµ„ÖŹ£¬¶žÕßÖŹĮæĻąµČŹ±ĘäĪļÖŹµÄĮæÖ®±ČĪŖ3 £ŗ2£»ZŌŖĖŲŌ×ӵēĪĶā²ćµē×ÓŹżŹĒĘä×īĶā²ćµē×ÓŹżµÄ4±¶”£Ōņ£ŗ
¢ŁŠ“³ö»ÆŗĻĪļ¼×µÄµē×ÓŹ½___________________£»
¢ŚŠ“³öZÓė¼×·“Ó¦µÄ»Æѧ·½³ĢŹ½__________________________________________________£»
£Ø2£©.X”¢Y”¢Z¶¼ŹĒ·Ē½šŹōµ„ÖŹ£¬XŹĒŌ×Ó¾§Ģ壬Y”¢Z¶¼ŹĒ·Ö×Ó¾§Ģ壬X”¢Y¶¼ÄÜÓėĒæ¼īČÜŅŗ·“Ó¦£»ŅŅµÄĖ®ČÜŅŗŹĒ¹¤ŅµČżĖįÖ®Ņ»£¬Ņ²ŹĒŹµŃéŹŅ³£ÓĆŹŌ¼Į”£Ōņ£ŗ
¢ŁŠ“³öXÓėNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½________________________________________£»
¢ŚZÓė¼×µÄ·“Ó¦µÄŅ»ÖÖÖŲŅŖ¹¤ŅµÓĆĶ¾ŹĒ___________________________________________£»
¢ŪŌŚ¢ŁĖłµĆČÜŅŗÖŠ¼ÓČėŅŅµÄČÜŅŗ£¬¹Ū²ģµ½µÄĻÖĻó______________________________________________________________________ £»
£Ø3£©.X”¢ZŹĒ³£¼ū½šŹō£¬ZÓė¼×µÄ·“Ó¦Ö»ÓŠŌŚøßĪĀĻĀ²ÅÄܽųŠŠ£¬¼×ŹĒŅ»ÖÖ¾ßÓŠ“ÅŠŌµÄ»ÆŗĻĪļ£¬ŅŅŌŚ¹¤ŅµÉĻ³£ÓĆÓŚÖĘČ”Zµ„ÖŹ”£Ōņ£ŗ
¢ŁŠ“³öŅŅÓėNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½_________________________________________________ £»
¢Ś½«¢ŁĖłµĆČÜŅŗÓėŠ”ĖÕ“ņČÜŅŗµÄ»ģŗĻ¹Ū²ģµ½µÄĻÖĻóŹĒ___________________________________ £»
¢Ū½«µČĪļÖŹµÄĮæµÄXŗĶZ·Ö±šÓė×ćĮæµÄĻ”ĮņĖį·“Ó¦£¬µ±Į½ÖÖ½šŹōĶźČ«Čܽāŗó£¬µĆµ½ĘųĢåµÄÖŹĮæÖ®±ČŹĒ____________________________”£
£ØĆææÕ2·Ö£¬¹²16·Ö£©£Ø1£©¢Ł 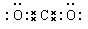 £» ¢Ś 2Mg+CO2
£» ¢Ś 2Mg+CO2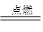
£Ø2£©¢ŁSi£«2OH££«H2O£½ SiO32££«2H2”ü £» ¢Ś ÖĘČ”øß“æ¹č£» ¢Ū²śÉś°×É«½ŗד³Įµķ£»
£Ø3£©¢ŁAl2O3£«2NaOH£½2NaAlO2£«H2O £» ¢Ś²śÉś°×É«½ŗד³Įµķ£» ¢Ū 2 : 3
”¾½āĪö”æ£Ø1£©øł¾ŻŌŖĖŲµÄŠŌÖŹ¼°½į¹¹æÉÖŖ£¬.X”¢Y”¢Z·Ö±šŹĒC”¢O”¢Mg”£
¢Ł¼×ŹĒCO2£¬ŗ¬ÓŠ¼«ŠŌ¼üµÄ¹²¼Ū»ÆŗĻĪļ£¬µē×ÓŹ½ĪŖ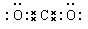 ”£
ӣ
¢ŚĆ¾ÄÜŌŚCO2ÖŠČ¼ÉÕ£¬Éś³ÉŃõ»ÆĆ¾ŗĶĢ¼£¬·½³ĢŹ½ĪŖ2Mg+CO2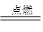
£Ø2£©ÄÜŗĶĒæ¼ī·“Ó¦µÄ·Ē½šŹōµ„ÖŹĒŅŹĒŌ×Ó¾§ĢåµÄÓ¦øĆŹĒ¹č£¬¼“XŹĒSi”£ŅŅµÄĖ®ČÜŅŗŹĒ¹¤ŅµČżĖįÖ®Ņ»£¬Ņ²ŹĒŹµŃéŹŅ³£ÓĆŹŌ¼Į£¬ĖłŅŌŅŅŹĒĀČ»ÆĒā£¬ŌņYŹĒĀČŌ×Ó£¬ZŹĒĒāŌŖĖŲ”£
¢Ł¹čŗĶĒāŃõ»ÆÄĘ·“Ӧɜ³É¹čĖįÄĘŗĶĒāĘų£¬·½³ĢŹ½ĪŖSi£«2OH££«H2O£½ SiO32££«2H2”ü”£
¢ŚĒāĘųŌŚøßĪĀĻĀŗ¬ÓŠĖÄĀČ»Æ¹čæÉŅŌÖĘČ”øß“æ¹č£¬
¢ŪŃĪĖįŹĒĒæĖį£¬ÄÜÖĘČ”¹čĖį£¬¹čĖį²»ČÜÓŚĖ®£¬Ņņ“ĖĻÖĻóŹĒ²śÉś°×É«½ŗד³Įµķ”£
£Ø3£©¼×ŹĒŅ»ÖÖ¾ßÓŠ“ÅŠŌµÄ»ÆŗĻĪļ£¬Ōņ¼×ŹĒĖÄŃõ»ÆČżĢś£¬ĖłŅŌZŹĒĀĮ£¬XŹĒĢś£¬ŅŅŹĒŃõ»ÆĀĮ£¬YŹĒŃõĘų”£
¢ŁŃõ»ÆĀĮŹĒĮ½ŠŌŃõ»ÆĪļ£¬ÄÜŗĶĒāŃõ»ÆÄĘČÜŅŗ·“Ӧɜ³ÉĘ«ĀĮĖįÄĘŗĶĖ®£¬·½³ĢŹ½ĪŖAl2O3£«2NaOH£½2NaAlO2£«H2O”£
¢ŚĘ«ĀĮĖįÄĘÄÜŗĶĢ¼ĖįĒāÄĘ·“Ӧɜ³ÉĒāŃõ»ÆĀĮ³ĮµķŗĶĢ¼ĖįÄĘ£¬ĖłŅŌĻÖĻóŹĒ²śÉś°×É«½ŗד³Įµķ”£
¢Ū1molĢśÄÜÉś³É1molĒāĘų£¬¶ų1molÄÜÉś³É1.5molĒāĘų£¬ĖłŅŌµĆµ½ĘųĢåµÄÖŹĮæÖ®±ČŹĒ2 : 3”£


 æĪĒ°æĪŗóĶ¬²½Į·Ļ°ĻµĮŠ“š°ø
æĪĒ°æĪŗóĶ¬²½Į·Ļ°ĻµĮŠ“š°ø æĪĢĆŠ”×÷ŅµĻµĮŠ“š°ø
æĪĢĆŠ”×÷ŅµĻµĮŠ“š°ø »ĘøŌŠ”דŌŖæŚĖćĖŁĖćĮ·Ļ°²įĻµĮŠ“š°ø
»ĘøŌŠ”דŌŖæŚĖćĖŁĖćĮ·Ļ°²įĻµĮŠ“š°ø ³É¹¦ŃµĮ·¼Ę»®ĻµĮŠ“š°ø
³É¹¦ŃµĮ·¼Ę»®ĻµĮŠ“š°ø ±¶ĖŁŃµĮ··ØÖ±ĶØÖŠæ¼æ¼µćĻµĮŠ“š°ø
±¶ĖŁŃµĮ··ØÖ±ĶØÖŠæ¼æ¼µćĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗŗÓ±±Ź”¼½ÖŻÖŠŃ§10-11ѧğøßŅ»ĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ£Ø»ÆѧĄķ£©B¾ķ ĢāŠĶ£ŗĢīæÕĢā
£ØĆææÕ2·Ö£¬¹²16·Ö£©ŅĄ¾ŻŹĀŹµ£¬ĢīæÕ£ŗ
£Ø1£©ŌŚ25”ę”¢101kPaĻĀ£¬1g¼×“¼ŅŗĢåĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬Ė®Ź±·ÅČČ22.68kJ”£Ōņ±ķŹ¾¼×“¼Č¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½ĪŖ_____________________________
£Ø2£©ŅŃÖŖ²šæŖ1molH£H¼ü£¬1molN£H¼ü£¬1molN”ŌN¼ü·Ö±šŠčŅŖµÄÄÜĮæŹĒ436kJ”¢391kJ”¢946kJ£¬ŌņN2ÓėH2·“Ӧɜ³ÉNH3µÄČČ»Æѧ·½³ĢŹ½ĪŖ_________________
£Ø3£©ĒāŃõČ¼ĮĻµē³ŲŅŃÓĆÓŚŗ½Ģģ·É»ś”£ŅŌ30%KOHČÜŅŗĪŖµē½āÖŹµÄÕāÖÖµē³ŲŌŚŹ¹ÓĆŹ±µÄµē¼«·“Ó¦Ź½£ŗ Õż¼«£ŗ £»øŗ¼«£ŗ
(4)ŌŚ25”ꏱ£¬ĆܱÕČŻĘ÷ÖŠX”¢Y”¢ZČżÖÖĘųĢåµÄ³õŹ¼ÅضČŗĶĘ½ŗāÅضČČēĻĀ±ķ£ŗ
| ĪļÖŹ | X | Y | Z |
| ³õŹ¼ÅضČ/mol”¤L-1 | 0.1 | 0.2 | 0 |
| Ę½ŗāÅضČ/mol”¤L-1 | 0.05 | 0.05 | 0.1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğŌĘÄĻŹ”ÓńĻŖŅ»ÖŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
£ØĆææÕ2·Ö£¬¹²16·Ö£©Ä³ŃŠ¾æŠŌѧĻ°Š”×éĪŖŗĻ³É1£¶”“¼£¬²éŌÄ׏ĮĻµĆÖŖŅ»ĢõŗĻ³ÉĀ·Ļߣŗ
CH3CH===CH2£«CO£«H2 CH3CH2CH2CHO
CH3CH2CH2CHO CH3CH2CH2CH2OH£ŗ
CH3CH2CH2CH2OH£ŗ
COµÄÖʱøŌĄķ£ŗHCOOH CO”ü£«H2O£¬²¢Éč¼Ę³öŌĮĻĘųµÄÖʱø×°ÖĆ(ČēĶ¼)”£
CO”ü£«H2O£¬²¢Éč¼Ę³öŌĮĻĘųµÄÖʱø×°ÖĆ(ČēĶ¼)”£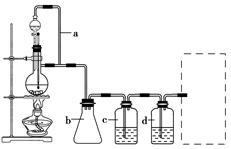
ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
(1)ŹµŃéŹŅĻÖÓŠŠæĮ£”¢Ļ”ĻõĖį”¢Ļ”ŃĪĖį”¢ÅØĮņĖį”¢2£±ū“¼£¬“ÓÖŠŃ”ŌńŗĻŹŹµÄŹŌ¼ĮÖʱø±ūĻ©”£Š“³ö»Æѧ·½³ĢŹ½£ŗ ____________________________________________________________________________”£
(2)ČōÓĆŅŌÉĻ×°ÖĆÖʱøH2£¬ŌŚŠéĻßæņÄŚ»³öŹÕ¼ÆøÉŌļH2µÄ×°ÖĆĶ¼”£
(3)ÖʱūĻ©Ź±£¬»¹²śÉśÉŁĮæSO2”¢CO2¼°Ė®ÕōĘų£¬øĆŠ”×éÓĆŅŌĻĀŹŌ¼Į¼ģŃéÕāĖÄÖÖĘųĢ壬»ģŗĻĘųĢåĶعżŹŌ¼ĮµÄĖ³ŠņŹĒ________(ĢīŠņŗÅ)
¢Ł±„ŗĶNa2SO3ČÜŅŗ””¢ŚĖįŠŌKMnO4ČÜŅŗ””¢ŪŹÆ»ŅĖ®””¢ÜĪŽĖ®CuSO4””¢ŻĘ·ŗģČÜŅŗ
(4)ŗĻ³ÉÕż¶”Č©µÄ·“Ó¦ĪŖÕżĻņ·ÅČȵÄæÉÄę·“Ó¦£¬ĪŖŌö“ó·“Ó¦ĖŁĀŹŗĶĢįøßŌĮĻĘųµÄ×Ŗ»ÆĀŹ£¬ÄćČĻĪŖÓ¦øĆ²ÉÓƵďŹŅĖ·“Ó¦Ģõ¼žŹĒ________”£
a£®µĶĪĀ”¢øßŃ¹”¢“߻ƼĮ b£®ŹŹµ±µÄĪĀ¶Č”¢øßŃ¹”¢“߻ƼĮ
c£®³£ĪĀ”¢³£Ń¹”¢“߻ƼĮ d£®ŹŹµ±µÄĪĀ¶Č”¢³£Ń¹”¢“߻ƼĮ
(5)Õż¶”Č©¾“߻ƼÓĒāµĆµ½ŗ¬ÉŁĮæÕż¶”Č©µÄ1£¶”“¼“ÖĘ·£¬ĪŖ“æ»Æ1£¶”“¼£¬øĆŠ”×é²éŌÄĪÄĻ×µĆÖŖ£ŗ¢ŁR”ŖCHO£«NaHSO3(±„ŗĶ)”śRCH(OH)SO3Na”ż £»¢Ś·Šµć£ŗŅŅĆŃ34”ę£¬1£¶”“¼118”ę£¬²¢Éč¼Ę³öČēĻĀĢį“æĀ·Ļߣŗ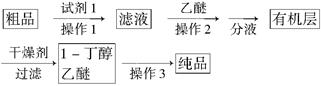
ŹŌ¼Į1ĪŖ________£¬²Ł×÷1ĪŖ________£¬²Ł×÷2ĪŖ________£¬²Ł×÷3ĪŖ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģŗžÄĻŹ”ĻęÖŠĆūŠ£øßČż9ŌĀµŚŅ»“ĪĮŖæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
£ØĆææÕ2·Ö£¬¹²16·Ö£©ĀĮĆ¾ŗĻ½šŅŃ³ÉĪŖ·É»śÖĘŌģ”¢»Æ¹¤Éś²śµČŠŠŅµµÄÖŲŅŖ²ÄĮĻ”£ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§£¬ĪŖ²ā¶Øijŗ¬Ć¾3£„-5£„µÄĀĮĆ¾ŗĻ½š£Ø²»ŗ¬ĘäĖüŌŖĖŲ£©ÖŠĆ¾µÄÖŹĮæ·ÖŹż£¬Éč¼ĘĻĀĮŠĮ½ÖÖ²»Ķ¬ŹµŃé·½°ø½ųŠŠĢ½¾æ”£ĢīŠ“ĻĀĮŠæÕ°×”£ [·½°øŅ»]
[·½°øŅ»] ”¼ŹµŃé·½°ø”½½«ĀĮĆ¾ŗĻ½šÓė×ćĮæNaOHČÜŅŗ·“Ó¦£¬²ā¶ØŹ£Óą¹ĢĢåÖŹĮ攣
”¼ŹµŃé·½°ø”½½«ĀĮĆ¾ŗĻ½šÓė×ćĮæNaOHČÜŅŗ·“Ó¦£¬²ā¶ØŹ£Óą¹ĢĢåÖŹĮ攣 ŹµŃéÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
ŹµŃéÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£ ”¼ŹµŃé²½Öč”½
”¼ŹµŃé²½Öč”½ £Ø1£©³ĘČ”5.4gĀĮĆ¾ŗĻ½š·Ūĩѳʷ£¬ČÜÓŚV mL 2.0 mol/L NaOHČÜŅŗÖŠ”£ĪŖŹ¹Ęä·“Ó¦ĶźČ«£¬ŌņNaOHČÜŅŗµÄĢå»żV ”Ż ”£
£Ø1£©³ĘČ”5.4gĀĮĆ¾ŗĻ½š·Ūĩѳʷ£¬ČÜÓŚV mL 2.0 mol/L NaOHČÜŅŗÖŠ”£ĪŖŹ¹Ęä·“Ó¦ĶźČ«£¬ŌņNaOHČÜŅŗµÄĢå»żV ”Ż ”£ £Ø2£©¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ”¢³ĘĮæ¹ĢĢ唣øĆ²½ÖčÖŠČōĪ“Ļ“µÓ¹ĢĢ壬²āµĆĆ¾µÄÖŹĮæ·ÖŹż½«
£Ø2£©¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ”¢³ĘĮæ¹ĢĢ唣øĆ²½ÖčÖŠČōĪ“Ļ“µÓ¹ĢĢ壬²āµĆĆ¾µÄÖŹĮæ·ÖŹż½«  (Ģī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±)”£
(Ģī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±)”£ [·½°ø¶ž
[·½°ø¶ž
 ]
]
”¼ŹµŃé·½°ø”½½«ĀĮĆ¾ŗĻ½šÓė×ćĮæĻ”ĮņĖįČÜŅŗ·“Ó¦£¬²ā¶ØÉś³ÉĘųĢåŌŚĶس£×“æö£ØŌ¼20”ę£¬1.01 105Pa£©µÄĢå»ż”£
105Pa£©µÄĢå»ż”£ ”¼ĪŹĢāĢÖĀŪ”½£Ø1£©Ķ¬Ń§ĆĒÄāŃ”ÓĆĻĀĮŠŹµŃé×°ÖĆĶź³ÉŹµŃé£ŗ
”¼ĪŹĢāĢÖĀŪ”½£Ø1£©Ķ¬Ń§ĆĒÄāŃ”ÓĆĻĀĮŠŹµŃé×°ÖĆĶź³ÉŹµŃé£ŗ





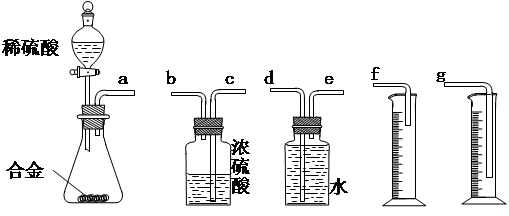

 ¢ŁÄćČĻĪŖ×ī¼ņŅ×µÄ×°ÖĆĘäĮ¬½ÓĖ³ŠņŹĒ£ŗa½Ó£Ø £©£Ø £©½Ó£Ø £©£Ø £©½Ó£Ø £©£ØĢī½ÓæŚ×ÖÄø£¬æɲ»ĢīĀś”££©
¢ŁÄćČĻĪŖ×ī¼ņŅ×µÄ×°ÖĆĘäĮ¬½ÓĖ³ŠņŹĒ£ŗa½Ó£Ø £©£Ø £©½Ó£Ø £©£Ø £©½Ó£Ø £©£ØĢī½ÓæŚ×ÖÄø£¬æɲ»ĢīĀś”££© ¢ŚŹµŃéæŖŹ¼Ź±£¬ĻČ“ņæŖ·ÖŅŗĀ©¶·ÉĻæŚµÄ²£Į§Čū£¬ŌŁĒįĒį“ņæŖ·ÖŅŗĀ©¶·æÉŠż×ŖµÄ»īČū£¬Ņ»»į¶łŗóĻ”ĮņĖįŅ²²»ÄÜĖ³ĄūµĪČė׶ŠĪĘæÖŠ”£ĒėÄć°ļÖś·ÖĪöŌŅņ ”£
¢ŚŹµŃéæŖŹ¼Ź±£¬ĻČ“ņæŖ·ÖŅŗĀ©¶·ÉĻæŚµÄ²£Į§Čū£¬ŌŁĒįĒį“ņæŖ·ÖŅŗĀ©¶·æÉŠż×ŖµÄ»īČū£¬Ņ»»į¶łŗóĻ”ĮņĖįŅ²²»ÄÜĖ³ĄūµĪČė׶ŠĪĘæÖŠ”£ĒėÄć°ļÖś·ÖĪöŌŅņ ”£ ¢ŪŹµŃé½įŹųŹ±£¬ŌŚ¶ĮČ”²āĮæŹµŃéÖŠÉś³ÉĒāĘųµÄĢå»żŹ±£¬ÄćČĻĪŖ×īŗĻĄķµÄĖ³ŠņŹĒ ”£
¢ŪŹµŃé½įŹųŹ±£¬ŌŚ¶ĮČ”²āĮæŹµŃéÖŠÉś³ÉĒāĘųµÄĢå»żŹ±£¬ÄćČĻĪŖ×īŗĻĄķµÄĖ³ŠņŹĒ ”£
A£®µČ“żŹµŃé×°ÖĆĄäČ“ | B£®ÉĻĻĀŅʶÆĮæĶ²f£¬Ź¹ĘäÖŠŅŗĆęÓė¹ćæŚĘæÖŠŅŗĆęĻąĘ½ | C£®ÉĻĻĀŅʶÆĮæĶ²g£¬Ź¹ĘäÖŠŅŗĆęÓė¹ćæŚĘæÖŠŅŗĆęĻąĘ½ | D£®ŹÓĻßÓė°¼ŅŗĆęµÄ×īµĶµćĖ®Ę½¶ĮČ”ĮæĶ²ÖŠĖ®µÄĢå»ż |
 £Ø2£©×ŠĻø·ÖĪöŹµŃé×°ÖĆŗó£¬Ķ¬Ń§ĆĒ¾ĢÖĀŪČĻĪŖŅŌĻĀĮ½µć»įŅżĘš½Ļ“óĪó²ī£ŗĻ”ĮņĖįµĪČė׶ŠĪĘæÖŠ£¬¼“Ź¹²»Éś³ÉĒāĘų£¬Ņ²»į½«ĘæÄŚæÕĘųÅųö£¬Ź¹Ėł²āĒāĘųĢå»żĘ«“ó£»ŹµŃé½įŹųŹ±£¬Į¬½Ó¹ćæŚĘæŗĶĮæĶ²µÄµ¼¹ÜÖŠÓŠÉŁĮæĖ®“ęŌŚ£¬Ź¹Ėł²āĒāĘųĢå»żĘ«Š””£ÓŚŹĒĖūĆĒÉč¼ĘĮĖÓŅĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ”£
£Ø2£©×ŠĻø·ÖĪöŹµŃé×°ÖĆŗó£¬Ķ¬Ń§ĆĒ¾ĢÖĀŪČĻĪŖŅŌĻĀĮ½µć»įŅżĘš½Ļ“óĪó²ī£ŗĻ”ĮņĖįµĪČė׶ŠĪĘæÖŠ£¬¼“Ź¹²»Éś³ÉĒāĘų£¬Ņ²»į½«ĘæÄŚæÕĘųÅųö£¬Ź¹Ėł²āĒāĘųĢå»żĘ«“ó£»ŹµŃé½įŹųŹ±£¬Į¬½Ó¹ćæŚĘæŗĶĮæĶ²µÄµ¼¹ÜÖŠÓŠÉŁĮæĖ®“ęŌŚ£¬Ź¹Ėł²āĒāĘųĢå»żĘ«Š””£ÓŚŹĒĖūĆĒÉč¼ĘĮĖÓŅĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ”£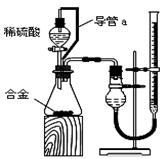
 ¢Ł×°ÖĆÖŠµ¼¹ÜaµÄ×÷ÓĆŹĒ ”£
¢Ł×°ÖĆÖŠµ¼¹ÜaµÄ×÷ÓĆŹĒ ”£ ¢ŚŹµŃéĒ°ŗó¼īŹ½µĪ¶Ø¹ÜÖŠŅŗĆę¶ĮŹż·Ö±šĪŖV1 mL”¢V2 mL”£Ōņ²śÉśĒāĘųµÄĢå»żĪŖ_________mL”£
¢ŚŹµŃéĒ°ŗó¼īŹ½µĪ¶Ø¹ÜÖŠŅŗĆę¶ĮŹż·Ö±šĪŖV1 mL”¢V2 mL”£Ōņ²śÉśĒāĘųµÄĢå»żĪŖ_________mL”£



²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğøŹĖąŹ”ĢģĖ®ŹŠøßČżÄ£Äā£Ø5ŌĀ£©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£ØĆææÕ2·Ö£¬¹²16·Ö£©¶ĢÖÜĘŚŌŖĖŲX”¢Y”¢Z”¢W£¬Ō×ÓŠņŹżŅĄ“ĪŌö“󔣳£ĪĀ³£Ń¹ĻĀ£¬Ö»ÓŠWµÄµ„ÖŹĪŖĘųĢ唣ĖüĆĒµÄ×īøßŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļŅĄ“ĪĪŖ¼×”¢ŅŅ”¢±ū”¢¶””£¼×”¢ŅŅ”¢±ūŹĒ֊ѧ»Æѧ֊µÄ³£¼ūĪļÖŹ£¬ĘäÖŠÖ»ÓŠŅŅÄŃČÜÓŚĖ®£¬ĒŅÄÜŗĶ¼×”¢±ū·“Ó¦µĆµ½³ĪĒåČÜŅŗ”£øł¾ŻŅŌÉĻŠÅĻ¢ĢīŠ“ĻĀĮŠæÕ°×£ŗ
¢Å»³öWµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼_________________________________________£»
¢Ę½«ŅŅŗĶ¼×”¢±ū·Ö±š·“Ó¦ŗóµĆµ½µÄČÜŅŗ»ģŗĻ£¬¹Ū²ģµ½µÄĻÖĻóŹĒ___________________
___________________ £¬Į½ČÜŅŗ»ģŗĻŹ±Ėł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________________________________________________£»
¢ĒĻĀĮŠŹĀŹµÄÜÖ¤Ć÷ZŗĶW·Ē½šŹōŠŌĒæČõµÄŹĒ£ØŃ”ĢīŠņŗÅ£©__________________________£»
A.µ„ÖŹµÄČŪµć£ŗZ£¾W2 B.ĖįŠŌ£ŗ¶”£¾±ū
C.ŌŚČÜŅŗÖŠ£ŗW2£«H2Z£½2HW£«Z
D.ĪČ¶ØŠŌ£ŗHW£¾H2Z E.Ēā»ÆĪļĖ®ČÜŅŗµÄĖįŠŌ£ŗHW£¾H2Z
F.ČܽāŠŌ£ŗ¶”£¾±ū
¢ČÓĆYµ„ÖŹŗĶÉś»īÖŠ×ī³£ÓĆµÄ½šŹō×÷µē¼«£¬ÓƵ¼ĻßĮ¬½Ó²åČė¼×µÄČÜŅŗÖŠ¹¹³ÉŌµē³Ų£¬øĆŌµē³Ųøŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ_______________________________________________ £»
¢É¹¤ŅµÉĻŅŌXWĪŖŌĮĻæÉŅŌ½ųŠŠŠķ¶ą»ÆÉś²ś£¬¼×ŗĶW2¶¼ŹĒĘäÖ÷ŅŖ²śĘ·”£Š“³ö¹¤ŅµÉĻŅŌXWĪŖŌĮĻÉś²ś¼×ŗĶW2µÄ»Æѧ·½³ĢŹ½_______________________________________________
_______________________________________________£»ČōŅŖÉś²ś80.0 kg¼×ĪļÖŹ£¬ÖĮÉŁŠčŅŖXW______________kg£¬Ķ¬Ź±æɵĆW2_____________m3£Ø±źæö£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com