
��4�֣�(1)ij��ѧ��ȤС����������װ����ѡȡ��Ҫ��װ����ȡ��NH4��2SO4��Һ�����ӵ�˳���ýӿ������ĸ��ʾ���ǣ�a

(2)��װ��C������Һ����뿪�IJ���������__ _______��
װ��D�������� ��
��4�֣�Ϊ����Ȼ�淋ľ��ü�ֵ���ҹ���ѧ�����������������þ�ȷֽ��Ȼ���ư������õ���ʽ�Ȼ�þ(MgOHCl)�Ĺ��ա�ijͬѧ���ݸ�ԭ����Ƶ�ʵ��װ����ͼ��

��ش��������⣺
(1) װ��A�з�����Ӧ���ɼ�ʽ�Ȼ�þ�Ļ�ѧ����ʽΪ_________ ____
װ��B�м�ʯ�ҵ�������_____ __
(2) ��Ӧ�����г���ͨ��N2�����������㣺һ��ʹ��Ӧ�����İ�����ȫ��������ϡ���������գ�����_______________ ______
(3) װ��C���Թ��з�Ӧ�����ӷ���ʽΪ ______________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣�(1)ij��ѧ��ȤС��ԡ�ũ��ɽȪ����Ȫˮ���м��ʱ������1.0 L�ÿ�Ȫˮ�к���45.6 mg Mg2������ÿ�Ȫˮ��Mg2�������ʵ���Ũ��Ϊ��������.
(2)��KCl��CaCl2����ɵ�ij������У�K����Ca2�������ʵ���֮��Ϊ2��1����KCl��CaCl2�����ʵ���֮��Ϊ�����������û������CaCl2����������Ϊ��������.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡׯ���е�������ѧ��һ��ѧ�ڵ�һ���¿���ѧ�� ���ͣ���ѡ��
��6�֣�(1)ij��ѧ��ȤС��ԡ�ũ��ɽȪ����Ȫˮ���м��ʱ������1.0 L�ÿ�Ȫˮ�к���45.6 mg Mg2������ÿ�Ȫˮ��Mg2�������ʵ���Ũ��Ϊ��������.
(2)��KCl��CaCl2����ɵ�ij������У�K����Ca2�������ʵ���֮��Ϊ2��1����KCl��CaCl2�����ʵ���֮��Ϊ�����������û������CaCl2����������Ϊ��������.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�����ʡ������ѧ��12���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
�� (1)ij��ѧ��ȤС����������װ����ѡȡ��Ҫ��װ����ȡ (NH4)2SO4��Һ�����ӵ�˳���ýӿ������ĸ��ʾ���ǣ�a
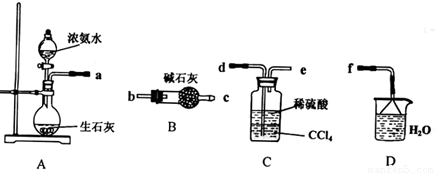
(2)��װ��C������Һ����뿪�IJ���������__ _______��
װ��D�������� ��
��4�֣�Ϊ����Ȼ�淋ľ��ü�ֵ���ҹ���ѧ�����������������þ�ȷֽ��Ȼ���ư������õ���ʽ�Ȼ�þ(MgOHCl)�Ĺ��ա�ijͬѧ���ݸ�ԭ����Ƶ�ʵ��װ����ͼ��
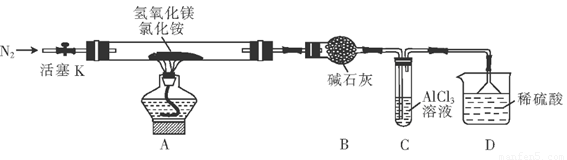
��ش��������⣺
(1) װ��A�з�����Ӧ���ɼ�ʽ�Ȼ�þ�Ļ�ѧ����ʽΪ_________ ____
װ��B�м�ʯ�ҵ�������_____ __
(2) ��Ӧ�����г���ͨ��N2�����������㣺һ��ʹ��Ӧ�����İ�����ȫ��������ϡ���������գ�����_______________ ______
(3) װ��C���Թ��з�Ӧ�����ӷ���ʽΪ ______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ��ɫ��У������һ��������ѧ�Ծ��������棩 ���ͣ�ʵ����
��.(1)ij��ѧ��ȤС����������װ����ѡȡ��Ҫ��װ����ȡ��NH4��2SO4��Һ�����ӵ�˳���ýӿ������ĸ��ʾ���ǣ�a ��
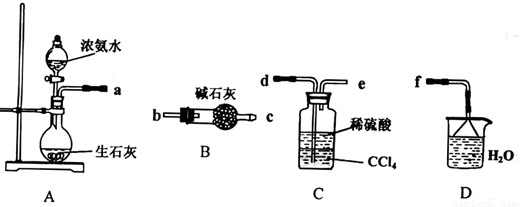
(2)��װ��C������Һ����뿪�IJ���������___ __��װ��D�������� ��
��.�������ƿ������ڸ��Ƶر�ˮ�ʡ��������ؽ������ӷ�ˮ�������ೱ��Ҳ������Ӧ�������ȡ���ҵ�������������Ƶ���Ҫ�������£�
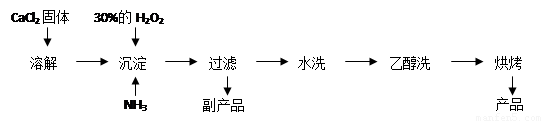
��֪CaO2��8H2O�ʰ�ɫ������ˮ��I2+2S2O32��= 2I��+S4O62��
��1��������������ȡCaO2��8H2O�Ļ�ѧ����ʽ�� ��
��2�����顰ˮϴ���Ƿ�ϸ�ķ����� ��
��3���ⶨ��Ʒ��CaO2�ĺ�����ʵ�鲽���ǣ�
��һ����ȷ��ȡa g��Ʒ����ƿ�У�������������ˮ������b g KI���壬�ٵ�������2 mol/L��H2SO4��Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊc mol��L��1��Na2S2O3��Һ����Ӧ��ȫ������Na2S2O3��ҺV mL��
���жϴ˵ζ�ʵ��ﵽ�յ�ķ����ǣ� ��
��CaO2����������Ϊ (����ĸ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡׯ���и�һ��ѧ�ڵ�һ���¿���ѧ�� ���ͣ�ѡ����
��6�֣�(1)ij��ѧ��ȤС��ԡ�ũ��ɽȪ����Ȫˮ���м��ʱ������1.0 L�ÿ�Ȫˮ�к���45.6 mg Mg2������ÿ�Ȫˮ��Mg2�������ʵ���Ũ��Ϊ��������.
(2)��KCl��CaCl2����ɵ�ij������У�K����Ca2�������ʵ���֮��Ϊ2��1����KCl��CaCl2�����ʵ���֮��Ϊ�����������û������CaCl2����������Ϊ��������.
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com