
��13�֣��ơ��ȼ��仯����������ת����ϵ���밴Ҫ����գ�
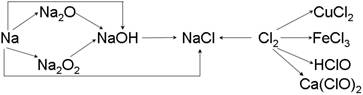
��1�������Ƶ��ܶȱ�ˮ ��ʵ�����н�����ͨ�������� �С�һС�������Ͷ��ˮ�еķ�Ӧ����ʽ�� ��
��2��Na2O2 �� ɫ�Ĺ��壬Na2O2 ����Ҫ��;�� ���йط�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ͨ������£�Ϊ�˷�ֹ��Ⱦ������������ʵ����������Ӧ��NaOH��Һ���գ��仯ѧ����ʽΪ������ ���� ��
��4��Ư�۵���Ч�ɷ��� ���ѧʽ����Ư������ˮ���ܿ����е�CO2���ã�������Ư�ס�ɱ�����õĴ����ᣬ��ѧ����ʽΪ ��
��13�֣���1��С ú�ͣ���1�֣� 2Na+ 2H2O��2NaOH+H2�� ��2�֣�
��2������ ��������� ����1�֣� 2Na2O2+2CO2=2Na2CO3+O2 ��2�֣�
��3��Cl2 +2NaOH=NaCl + NaClO + H2O��2�֣�
��4��Ca(ClO)2 ��1�֣� Ca(ClO)2 + CO2 + H2O == CaCO3��+ 2 HClO ��2�֣�
�������������ơ��������仯��������ʺ��й���;��
��1�������Ƶ��ܶ�С��ˮ�ģ����ڽ����Ƽ��ױ���������ˮ������Ӧ�ò�����ú���С��ƺ�ˮ��Ӧ��������������������Ӧ�ķ���ʽ��2Na+ 2H2O��2NaOH+H2�� ��
��2�����������ǵ���ɫ���壬����Ҫ����;����������ߵĹ���������Ӧ�ķ���ʽ��2Na2O2+2CO2=2Na2CO3+O2��2Na2O2+2H2O=4NaOH+O2��
��3�������ж�����Ҫβ����������Ӧ�ķ���ʽ��Cl2 +2NaOH=NaCl + NaClO + H2O��
��4��Ư���ǻ�����Ҫ�ɷ��Ǵ�����ƺ��Ȼ��ƣ�����Ч�ɷ��Ǵ�����ƣ�̼�������ǿ�ڴ�����ģ����Դ������������CO2��ˮ�������ɴ����ᣬ��Ӧ�ķ���ʽ��Ca(ClO)2 + CO2 + H2O == CaCO3��+ 2 HClO��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
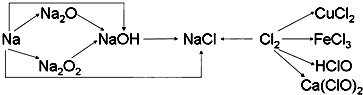
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ����һ�и�һ��һ���¿���ѧ�Ծ����������� ���ͣ������
��13�֣��ơ��ȼ��仯����������ת����ϵ���밴Ҫ����գ�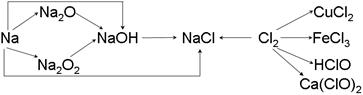
��1�������Ƶ��ܶȱ�ˮ ��ʵ�����н�����ͨ�������� �С�һС�������Ͷ��ˮ�еķ�Ӧ����ʽ�� ��
��2��Na2O2 �� ɫ�Ĺ��壬Na2O2 ����Ҫ��;�� ���йط�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ͨ������£�Ϊ�˷�ֹ��Ⱦ������������ʵ����������Ӧ��NaOH��Һ���գ��仯ѧ����ʽΪ������ ���� ��
��4��Ư�۵���Ч�ɷ��� ���ѧʽ����Ư������ˮ���ܿ����е�CO2���ã�������Ư�ס�ɱ�����õĴ����ᣬ��ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ��һ��ѧ����ĩ���⻯ѧ�������Ծ� ���ͣ������
(16��) ���仯����������ת����ϵ
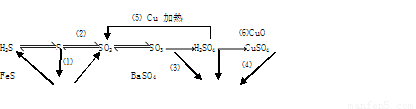
(1)����������ԭ��Ӧ���� (�����)
(2)д��(5)�ķ�Ӧ����ʽ ��
˵��Ũ������� ����ȡCuSO4�� �������(�����)��
(3)SO2����ɿ�����Ⱦ���γ��������Ҫ���ʡ�SO2��ˮ�Ĵ�������������Ӧ�������ᣬ��Ӧ����ʽΪ ��
��֤��������ķ����� ��
(4)ij��Һ�к���Cl-��SO42-�����ܺ���Na+��Fe2+������һ�֡�
����֤Cl-��SO42-�ķ�����
A.�ȼ�BaCl2��Һ���ȳ������ټ�AgNO3��Һ
B.�ȼ�AgNO3��Һ���ȳ������ټ�BaCl2��Һ
C.�ȼ�Ba(NO3)2��Һ���ȳ������ټ�AgNO3��Һ
����֤Na+��Fe2+��ķ����� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com