
��ͼ��һЩ�����ĵ��ʡ�������֮���ת����ϵͼ����Щ��Ӧ�еIJ������ʱ���ȥ����Ӧ�ٳ���Ӧ����Ұ�⺸�Ӹֹ죬���ǹ�ҵ����Ҫ�ķ�Ӧ֮һ��
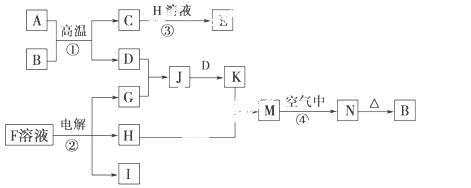
��ش��������⣺
(1)H�ĵ���ʽ��________________�����к��еĻ�ѧ��������___________
_________________��
(2)д����Ӧ�ܵ�����_____________________________________________
__________________________________________��
�йط�Ӧ�Ļ�ѧ����ʽΪ____________________________________________
___________________________��
(3)��֪I��ȼ�����ǣ�285.8 kJ��mol��1����1 m3(��״��)I��ȫȼ�գ��ָ�������ʱ�ų���������________(����������3λ��Ч����)��
(4)25 ��ʱ����PtΪ�缫��⺬���� ����̪��F�ı�����Һ������________(�����������)��������Һ����ɫ��Ϊ��ɫ�����ڴ˼��ռ���0.2 g���壬���ʱ��Һ��pH��________(������Һ�����Ϊ2 L�Ҳ����ǵ�����Һ����ı仯)��
����̪��F�ı�����Һ������________(�����������)��������Һ����ɫ��Ϊ��ɫ�����ڴ˼��ռ���0.2 g���壬���ʱ��Һ��pH��________(������Һ�����Ϊ2 L�Ҳ����ǵ�����Һ����ı仯)��
(5)��K��Һ�м�����K�����ʵ�����Na2O2��ǡ��ʹKת��ΪN��д���÷�Ӧ�����ӷ���ʽ��_________________________________________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�ѧʽֻ����һ�����ʷ��ӵ��� �� ��
A��CH2Cl2 B��C2H4Cl2 C��C4H10 D��C2H6O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
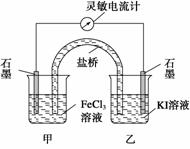
�����ʺϵ�����������Ӧ2Fe3+��2I�� 2Fe2+��I2��Ƴ�����ͼ��ʾ��ԭ��ء������жϲ���ȷ���ǣ� ��
2Fe2+��I2��Ƴ�����ͼ��ʾ��ԭ��ء������жϲ���ȷ���ǣ� ��
A����Ӧ��ʼʱ������ʯī�缫�Ϸ���������Ӧ
B����Ӧ��ʼʱ������ʯī�缫�ϵ�Fe3������ԭ
C�������ƶ���Ϊ��ʱ����Ӧ�ﵽ��ѧƽ��״̬
D�������ƶ���Ϊ����ڼ�������FeCl2���壬���е�ʯī�缫Ϊ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ŷ�ӦFe(s)+CO2(g) FeO(s)+CO(g) ��H1��ƽ�ⳣ��ΪK1��
FeO(s)+CO(g) ��H1��ƽ�ⳣ��ΪK1��
��ӦFe(s)+H2O(g) FeO(s)+H2(g) ��H2��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ���±���
FeO(s)+H2(g) ��H2��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ���±���
| 700�� | 900�� | |
| K1 | 1��47 | 2��15 |
| K2 | 2��38 | 1��67 |
�ٷ�ӦCO2(g) + H2(g) CO(g) + H2O(g) ��H��ƽ�ⳣ��ΪK�����H= ���á�H1�͡�H2��ʾ����K= ����K1��K2��ʾ�����������������֪����ӦCO2(g) + H2(g)
CO(g) + H2O(g) ��H��ƽ�ⳣ��ΪK�����H= ���á�H1�͡�H2��ʾ����K= ����K1��K2��ʾ�����������������֪����ӦCO2(g) + H2(g) CO(g) + H2O(g)�� ��Ӧ������ȡ����ȡ�����
CO(g) + H2O(g)�� ��Ӧ������ȡ����ȡ�����
�����ж�CO2(g) + H2(g) CO(g) + H2O(g)�ﵽ��ѧƽ��״̬�������� ������ţ���
CO(g) + H2O(g)�ﵽ��ѧƽ��״̬�������� ������ţ���
A��������ѹǿ���� B�����������c(CO)����
C��v��(H2)= v��(H2O) D��c(CO)= c(CO2)
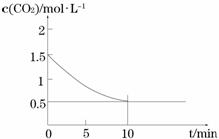 ��һ���¶��£���ij�ܱ������м����������۲�����һ������CO2���壬������ӦFe(s)+CO2(g)
��һ���¶��£���ij�ܱ������м����������۲�����һ������CO2���壬������ӦFe(s)+CO2(g) FeO(s)+CO(g) ��H > 0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
FeO(s)+CO(g) ��H > 0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
�� �������·�Ӧ��ƽ�ⳣ��Ϊ ��������������CO2����ʼŨ��Ϊ2.0 mol��L��1����ƽ��ʱCO2��Ũ��Ϊ_________mol��L��1��
�����д�ʩ����ʹƽ��ʱ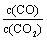 �������______������ţ���
�������______������ţ���
A�������¶� B������ѹǿ
C������һ������CO2 D���ټ���һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ������֮���ת����ϵ���� ֪D��E��Z����ѧ��ѧ�����ĵ��ʣ��������ǻ����Z��Y���ȼҵ�IJ�Ʒ��DԪ
֪D��E��Z����ѧ��ѧ�����ĵ��ʣ��������ǻ����Z��Y���ȼҵ�IJ�Ʒ��DԪ �ص�ԭ����������������Ӳ�����ȣ���D�������ο�����ˮ����EΪ�ճ�������Ӧ����㷺�Ľ���������Ӧ���⣬������Ӧ����ˮ��Һ�н��С���ش��������⡣
�ص�ԭ����������������Ӳ�����ȣ���D�������ο�����ˮ����EΪ�ճ�������Ӧ����㷺�Ľ���������Ӧ���⣬������Ӧ����ˮ��Һ�н��С���ش��������⡣
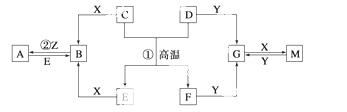
(1)д���������ʵĻ�ѧʽ��B___ _________��G___________________��Y______________��
_________��G___________________��Y______________��
(2)�ڵ�ƹ�ҵ�У�����E��Ϊ���ƽ�����ͭΪ�Ʋ��������E��__________������д���ڴ˵缫�Ϸ����ĵ缫��Ӧʽ�� ________________��
(3)д����Ӧ�ٵĻ�ѧ����ʽ___________��
(4)A��Һ��NaOH��Һ��Ͽ��γɳ�����ij�¶��´˳�����Ksp��2.097��10��39����
0.01 mol��L��1��A��Һ��0.001 mol��L��1��NaOH��Һ�������ϣ�����Ϊ�ܷ��γɳ���_______(��ܡ����ܡ�)����ͨ������˵��_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B��C��D��E����ѧ��ѧ�г��������ֻ�������Ƿֱ��ɶ���������Ԫ����ɣ��ס��ҡ����ǵ��ʣ������¼ס�����A��C��D��E�����壬BΪҺ�壬��Ϊ���壬�٢۱����ڸ����·�Ӧ���ܷ�������Щ���ʺͻ�����֮��������¹�ϵ��
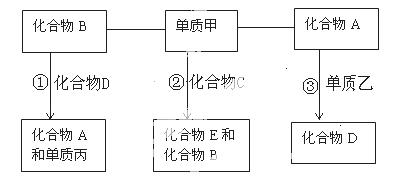
��1��д���ķ���ʽ ; A�Ľṹʽ ��B�ĵ���ʽ ��
��2������Ӧ������Ҫ�Ĺ�ҵ��Ӧ����÷�Ӧ�Ļ�ѧ����ʽΪ ��
����Ӧ�����ɵĻ�����E����Ư���ԣ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��1mol�����Һ�1mol������B�ڸ����·�Ӧ������1mol������D��1mol���ʱ�������131.5kJ������д���䷴Ӧ���Ȼ�ѧ����ʽ�� ��
��4�����ܱ������У���Ӧ�����ڸ����²��ܷ����Ŀ��淴Ӧ���仯ѧ��Ӧ����ʽΪ��
����Ӧ��ƽ�����������ϵ��ѹǿ����ƽ�� ���������ƶ��������ƶ������ƶ������������ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijЩ��Һ�к��е�������һ�㷽�����Գ�ȥ�����õ�ⷨ����һ�ֺ���Ч�ķ������������ʣ�������Ϊ���ʣ������ö��Ե缫��ⷨ��ȥ���ǣ� ��
A. Na2SO4��Һ��CuSO4�� B. NaNO3��Һ��CuCl2��
C. MgSO4��Һ��CuCl2�� D. NaNO3��Һ��MgCl2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ʵ���֮��Ϊ2��5��п��ϡ���ᷴӦ�������ᱻ��ԭ�IJ���ΪN2O����Ӧ������пû��ʣ�࣬��÷�Ӧ�б���ԭ��������δ����ԭ����������ʵ���֮����
A. 1��4 B.1��5 C. 2��3 D.2��5
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com