
| ¢Ł | ¢Ś | ¢Ū | ¢Ü | ¢Ż |
| ĪļÖŹĖłŗ¬µÄĪ¢Į£Źż | ¹ĢĢåĢå»ż | ČÜŅŗµÄÖŹĮæ°Ł·Ö±ČÅØ¶Č | ±ź×¼×“æöĻĀĘųĢåµÄĦ¶ūĢå»ż | ·Ē±ź×¼×“æöĻĀijĪļÖŹµÄÖŹĮæ |
| °¢·ü¼ÓµĀĀŽ³£Źż | ¹ĢĢåĆÜ¶Č | ČÜŅŗĢå»ż | ±ź×¼×“æöĻĀĘųĢåµÄĢå»ż | ĪļÖŹµÄĦ¶ūÖŹĮæ |
·ÖĪö £Ø1£©øł¾Żn=$\frac{N}{{N}_{A}}$=$\frac{V}{{V}_{m}}$=$\frac{m}{M}$=cV¼ĘĖć£»
£Ø2£©øł¾Żn=$\frac{N}{{N}_{A}}$=$\frac{V}{{V}_{m}}$=$\frac{m}{M}$¼ĘĖć£¬ĻąĶ¬Ģõ¼žĻĀ£¬ĘųĢåµÄĆܶČÓėĦ¶ūÖŹĮæ³ÉÕż±Č£¬½įŗĻ·Ö×ӵĹ¹³É·ÖĪö½ā“š£®
½ā“š ½ā£ŗ£Ø1£©¢ŁŅŃÖŖĪļÖŹĪ¢Į£ŹżŗĶ°¢·ü¼ÓµĀĀŽ³£Źż£¬øł¾Żn=$\frac{N}{{N}_{A}}$æɼĘĖćĪļÖŹµÄĮ棬¹Ź¢ŁÕżČ·£»
¢ŚŅŃÖŖ¹ĢĢåµÄĆܶČŗĶ¹ĢĢåĢå»ż£¬æɼĘĖć¹ĢĢåÖŹĮ棬²»ÄܼĘĖćĪļÖŹµÄĮ棬¹Ź¢Ś“ķĪó£»
¢ŪŅŃÖŖČÜŅŗµÄÖŹĮæ°Ł·Ö±ČÅضČŗĶČÜŅŗĢå»ż£¬²»ÄÜČ·¶ØĪļÖŹµÄĮæÅضČŗĶĪļÖŹµÄĮ棬¹Ź¢Ū“ķĪó£»
¢ÜŅŃÖŖ±ź×¼×“æöĻĀĘųĢåµÄĦ¶ūĢå»żŗĶ±ź×¼×“æöĻĀĘųĢåµÄĢå»ż£¬øł¾Żn=$\frac{V}{{V}_{m}}$£¬æɼĘĖćĪļÖŹµÄĮ棬¹Ź¢ÜÕżČ·£»
¢ŻŅŃÖŖ·Ē±ź×¼×“æöĻĀijĪļÖŹµÄÖŹĮæŗĶĪļÖŹµÄĦ¶ūÖŹĮ棬øł¾Żn=$\frac{m}{M}$æɼĘĖćĪļÖŹµÄĮ棬¹Ź¢ŻÕżČ·£®
¹Ź“š°øĪŖ£ŗ¢Ł¢Ü¢Ż£»
£Ø2£©¢Ł6.72L CH4 ĪļÖŹµÄĮæĪŖ$\frac{6.72L}{22.4L/mol}$=0.3mol£¬
¢Ś3.01”Į1023øöHCl·Ö×ÓµÄĪļÖŹµÄĮæĪŖ0.5mol£¬
¢Ū13.6g H2S µÄĪļÖŹµÄĮæĪŖ$\frac{13.6g}{34g/mol}$=0.4mol£¬
¢Ü0.2mol NH3£®
A£®ĻąĶ¬Ģõ¼žĻĀ£¬ĘųĢåµÄĢå»żÖ®±ČµČÓŚĪļÖŹµÄĮæÖ®±Č£¬ĖłŅŌĢå»żĢå»ż¢Ś£¾¢Ū£¾¢Ł£¾¢Ü£¬¹ŹAÕżČ·£»
B£®ø÷ĪļÖŹµÄĦ¶ūÖŹĮæ·Ö±šĪŖ¢ŁCH4 ĪŖ16g/mol¢ŚHClĪŖ36.5g/mol ¢ŪH2S ĪŖ34g/mol¢ÜNH3ĪŖ17g/mol£¬ĻąĶ¬Ģõ¼žĻĀ£¬ĆܶČÖ®±ČµČӌĦ¶ūÖŹĮæÖ®±Č£¬ĖłŅŌĆÜ¶Č¢Ś£¾¢Ū£¾¢Ü£¾¢Ł£¬¹ŹBÕżČ·£»
C£®ø÷ĪļÖŹµÄÖŹĮæ·Ö±šĪŖ¢ŁCH4 ĪŖ0.3mol”Į16g/mol=4.8g£¬¢ŚHClĪŖ0.5mol”Į36.5g/mol=18.25g£¬¢ŪH2S 13.6g£¬¢ÜNH3ĪŖ0.2mol”Į17g/mol=3.4g£¬ĖłŅŌÖŹĮæ¢Ś£¾¢Ū£¾¢Ł£¾¢Ü£¬¹ŹCÕżČ·£»
D£®ø÷ĪļÖŹÖŠHŌ×ÓµÄĪļÖŹµÄĮæ·Ö±šĪŖ¢ŁCH4 ĪŖ0.3mol”Į4=1.2mol¢ŚHClĪŖ0.5mol¢ŪH2S 0.4mol”Į2=0.8mol¢ÜNH3ĪŖ0.2mol”Į3=0.6mol£¬ĖłŅŌĒāŌ×ÓøöŹż¢Ł£¾¢Ū£¾¢Ü£¾¢Ś£¬¹ŹDÕżČ·£»
¹Ź“š°øĪŖ£ŗABCD£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹµÄĮæµÄÓŠ¹Ų¼ĘĖć£¬ÄŚČŻÉę¼°³£ÓĆ»Æѧ¼ĘĮæÓŠ¹Ų¼ĘĖć”¢°¢·üŁ¤µĀĀŽ¶ØĀɼ°ĶĘĀŪ£¬ÄŃ¶Č²»“ó£¬Ö¼ŌŚæ¼²éѧɜ¶Ō»ł“”ÖŖŹ¶µÄÕĘĪÕ£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | F | B£® | N | C£® | O | D£® | H |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na+”¢K+”¢HCO3-”¢Cl- | B£® | K+”¢NH4+”¢Cl-”¢SO42- | ||
| C£® | K+”¢Cu2+”¢SO42”¢Cl- | D£® | Mg2+”¢Cu2+”¢Cl-”¢NO3- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŌŚŗ¬ÓŠNAøöCH3COO-µÄ“×ĖįČÜŅŗÖŠ£¬H+ŹżÄæĀŌ“óÓŚNA | |
| B£® | 1molCl2ŗĶ×ćĮæĒāŃõ»ÆÄĘČÜŅŗ³ä·Ö·“Ó¦£¬×ŖŅʵē×ÓŹżÄæĪŖ2NA | |
| C£® | µē½ā¾«Į¶ĶŹ±£¬Čō×ŖŅĘĮĖNAøöµē×Ó£¬ŌņŃō¼«ÖŹĮæ¼õŠ”32g | |
| D£® | ±źæöĻĀ£¬11.2LSO3Ėłŗ¬µÄ·Ö×ÓŹżÄæĪŖ0.5NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Éś³É0.025 mol P2O5 | |
| B£® | Éś³É P2O3ĖłŹĶ·ÅµÄČČĮæĪŖ£ØY-0.05X£© kJ | |
| C£® | 2P£Øs£©+$\frac{3}{2}$O2£Øg£©=P2O3£Øs£©”÷H=-£Ø40Y-2X£©kJ•mol-1 | |
| D£® | Éś³ÉµÄ P2O3Óė P2O5µÄÖŹĮæÖ®±ČĪŖ 1£ŗ1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
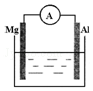 ČēĶ¼ĖłŹ¾×°ÖĆÖŠ£¬½šŹōĆ¾”¢ĀĮŗĶµēĮ÷±ķĶعżµ¼ĻßĻąĮ¬£ŗ
ČēĶ¼ĖłŹ¾×°ÖĆÖŠ£¬½šŹōĆ¾”¢ĀĮŗĶµēĮ÷±ķĶعżµ¼ĻßĻąĮ¬£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
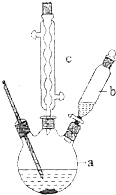 ±½ŅŅĖįŹĒÓŠ»śŗĻ³ÉµÄÖŠ¼ä²śĪļ£¬ĻĀĆęŹĒĖüµÄŅ»ÖÖŹµŃéŹŅŗĻ³ÉĀ·Ļߣŗ
±½ŅŅĖįŹĒÓŠ»śŗĻ³ÉµÄÖŠ¼ä²śĪļ£¬ĻĀĆęŹĒĖüµÄŅ»ÖÖŹµŃéŹŅŗĻ³ÉĀ·Ļߣŗ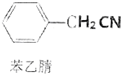 +H2O+H2SO4$\stackrel{100”«130”ę}{”ś}$
+H2O+H2SO4$\stackrel{100”«130”ę}{”ś}$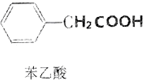 +NH4HSO4
+NH4HSO4²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com