
·ÖĪö £Ø1£©ŅĄ¾Żm=cVM¼ĘĖćŠčŅŖČÜÖŹµÄÖŹĮ棻
£Ø2£©øł¾ŻŹµŃé²Ł×÷µÄ²½ÖčŅŌ¼°Ćæ²½²Ł×÷ŠčŅŖŅĒĘ÷Č·¶Ø·“Ó¦ĖłŠčŅĒĘ÷·ÖĪö£»
£Ø3£©ĒāŃõ»ÆÄĘ¾ßÓŠøÆŹ“ŠŌ£»
£Ø4£©øł¾ŻČÜŅŗµÄÅäÖĘ²Ł×÷·ÖĪö£»
£Ø5£©·ÖĪö²»µ±²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗµÄĢå»żµÄÓ°Ļģ£¬ŅĄ¾ŻC=$\frac{n}{V}$½ųŠŠĪó²ī·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©ÅäÖĘ0.1mol/LµÄNaOHČÜŅŗ480mL£¬Ó¦Ń”Ōń500mlČŻĮæĘ棬ÅäÖĘ500mlČÜŅŗ£¬ŠčŅŖĒāŃõ»ÆÄʵÄÖŹĮæm=0.1mol/L”Į0.5L”Į40g/mol=2.0g£»
¹Ź“š°øĪŖ£ŗ2.0£»
£Ø2£©ÅäÖĘĖ³ŠņŹĒ£ŗ¼ĘĖ攜³ĘĮæ”śČܽā”¢ĄäČ“”śŅĘŅŗ”śĻ“µÓ”ś¶ØČŻ”śŅ”ŌČ”ś×°ĘæĢłĒ©£¬Ņ»°ćÓĆĢģĘ½³ĘĮæ£ØÓƵ½Ņ©³×£©³ĘĮ棬ŌŚÉÕ±ÖŠČܽā£¬ĄäČ“ŗó×ŖŅʵ½500mLČŻĮæĘæÖŠ£¬²¢ÓĆ²£Į§°ōŅżĮ÷£¬×ŖŅĘĶź±Ļ£¬ÓĆÉŁĮæÕōĮóĖ®Ļ“µÓÉÕ±¼°²£Į§°ō2”«3“Ī²¢½«Ļ“µÓŅŗČ«²æ×ŖŅʵ½ČŻĮæĘæÖŠ£¬ŌŁ¼ÓŹŹĮæÕōĮóĖ®£¬µ±¼ÓĖ®ÖĮŅŗĆę¾ąĄėæĢ¶ČĻß1”«2cmŹ±£¬øÄÓĆ½ŗĶ·µĪ¹ÜµĪ¼Ó£¬Ź¹ČÜŅŗµÄ°¼ŅŗĆęµÄ×īµĶµćÓėæĢĻßĻąĘ½£¬ČūŗĆĘæČū£¬·“ø“ÉĻĻĀµßµ¹Ņ”ŌČ£®ĖłŅŌŠčŅŖµÄŅĒĘ÷ĪŖ£ŗĶŠÅĢĢģĘ½”¢Ņ©³×”¢ÉÕ±”¢²£Į§°ō”¢500mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü£¬
±ŲŠėŹ¹ÓƵÄŅĒĘ÷ÓŠ£ŗ¢ŁÉÕ± ¢ŚŅ©³× ¢Ü500mLČŻĮæĘæ ¢ŻĶŠÅĢĢģĘ½¢Ž²£Į§°ō£»
»¹ŠčŅŖµÄŅĒĘ÷ÓŠ£ŗ½ŗĶ·µĪ¹Ü£»
¹Ź“š°øĪŖ£ŗ¢Ł¢Ś¢Ü¢Ż¢Ž£»½ŗĶ·µĪ¹Ü£»
£Ø3£©ĒāŃõ»ÆÄĘ¾ßÓŠøÆŹ“ŠŌ£¬Ó¦·ÅŌŚŠ”ÉÕ±ÖŠ³ĘĮ棬¹Ź“š°øĪŖ£ŗ¢Ū£»
£Ø4£©ÅäÖĘŹ±£¬Ņ»°ć·ÖĪŖŅŌĻĀ¼øøö²½Öč£ŗ
¢Ś¼ĘĖć ¢Ł³ĘĮæ ¢ŪČܽā ¢ąĄäČ“ ¢Ż×ŖŅĘ ¢ŽĻ“µÓ ¢ß¶ØČŻ ¢ÜŅ”ŌČ£¬Ōņ²Ł×÷Ė³ŠņĪŖ£ŗ¢Ś¢Ł¢Ū¢ą¢Ż¢Ž¢ß¢Ü£»
¹Ź“š°øĪŖ£ŗB£»
£Ø5£©¢ŁĪ“Ļ“µÓÉÕ±”¢²£Į§°ō£¬µ¼ÖĀČÜÖŹµÄĪļÖŹµÄĮæĘ«Š”£¬ČÜŅŗµÄÅضČĘ«µĶ£¬¹Ź²»Ń”£»
¢ŚNaOHČÜŅŗĪ“ĄäČ“ÖĮŹŅĪĀ¾Ķ×ŖŅʵ½ČŻĮæĘæÖŠ£¬ĄäČ“ŗóČÜŅŗµÄĢå»żŠ”ÓŚ500ml£¬ČÜŅŗµÄÅضČĘ«øߣ¬¹ŹŃ”£»
¢ŪČŻĮæĘæ²»øÉŌļ£¬ŗ¬ÓŠÉŁĮæÕōĮóĖ®£¬¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗµÄĢå»ż¶¼²»»į²śÉśÓ°Ļģ£¬ČÜŅŗÅØ¶Č²»±ä£¬¹Ź²»Ń”£»
¢Ü¶ØČŻŹ±ø©ŹÓæĢ¶Č£¬µ¼ÖĀČÜŅŗµÄĢå»żĘ«Š”£¬ČÜŅŗµÄÅضČĘ«øߣ¬¹ŹŃ”£»
¹ŹŃ”£ŗ¢Ś¢Ü£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ”¢ŅĒĘ÷µÄŹ¹ÓĆŅŌ¼°Īó²ī·ÖĪö£¬Ć÷Č·ÅäÖĘŌĄķ¼°²Ł×÷¹ż³ĢŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄŃ¶Č²»“󣬲ąÖŲÓŚæ¼²éѧɜµÄ·ÖĪöÄÜĮ¦ŗĶŹµŃé²Ł×÷ÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ·“Ó¦Ź±¼ä/min | n£ØCO£©/mol | n£ØH2O£©/mol | n£ØCO2£©/mol | n£ØH2£©/mol |
| 0 | 1.20 | 0.60 | 0 | 0 |
| t1 | 0.80 | |||
| t2 | 0.20 |
| A£® | ·“Ó¦ŌŚt1minÄ©µÄĖŁĀŹĪŖv£ØH2£©=0.2/t mol•L-1•min-1 | |
| B£® | Ę½ŗāŹ±Ė®ÕōĘųµÄ×Ŗ»ÆĀŹĪŖ66.67% | |
| C£® | øĆĪĀ¶ČĻĀ·“Ó¦µÄĘ½ŗā³£ŹżĪŖl | |
| D£® | ĘäĖūĢõ¼ž²»±ä£¬ČōĘšŹ¼Ź±£¬n£ØCO£©=0.60 mol£¬n£ØH2O£©=1.20 mol£¬ŌņĘ½ŗāŹ±Ė®ÕōĘųµÄ×Ŗ»ÆĀŹĪŖ33.33% |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | $\frac{200a}{m}$mol-1 | B£® | $\frac{200m}{a}$mol-1 | C£® | $\frac{2m}{a}$mol-1 | D£® | $\frac{2a}{m}$mol-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
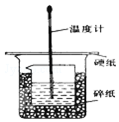 ijŹµŃ銔×éÉč¼ĘÓĆ50mL 1.0mol/LŃĪĖįøś50mL 1.1mol/L ĒāŃõ»ÆÄĘČÜŅŗŌŚČēĶ¼×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦£®ŌŚ“óÉÕ±µ×²æµęĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬Ź¹·ÅČėµÄŠ”ÉÕ±±æŚÓė“óÉÕ±±æŚĻąĘ½£®Č»ŗóŌŁŌŚ“󔢊”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬“óÉÕ±ÉĻÓĆÅŻÄĖÜĮĻ°å£Ø»ņÓ²Ö½°å£©×÷øĒ°å£¬ŌŚ°åÖŠ¼äæŖĮ½øöŠ”æ×£¬ÕżŗĆŹ¹ĪĀ¶Č¼ĘŗĶ»·ŠĪ²£Į§½Į°č°ōĶعż£®Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
ijŹµŃ銔×éÉč¼ĘÓĆ50mL 1.0mol/LŃĪĖįøś50mL 1.1mol/L ĒāŃõ»ÆÄĘČÜŅŗŌŚČēĶ¼×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦£®ŌŚ“óÉÕ±µ×²æµęĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬Ź¹·ÅČėµÄŠ”ÉÕ±±æŚÓė“óÉÕ±±æŚĻąĘ½£®Č»ŗóŌŁŌŚ“󔢊”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬“óÉÕ±ÉĻÓĆÅŻÄĖÜĮĻ°å£Ø»ņÓ²Ö½°å£©×÷øĒ°å£¬ŌŚ°åÖŠ¼äæŖĮ½øöŠ”æ×£¬ÕżŗĆŹ¹ĪĀ¶Č¼ĘŗĶ»·ŠĪ²£Į§½Į°č°ōĶعż£®Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ| ŹµŃéŠņŗÅ | ĘšŹ¼ĪĀÖŻ t1/”ę | ÖÕÖ¹ĪĀ¶Č£Øt2£©/”ę[ | ĪĀ²ī £Øt2-t1£©/”ę | ||
| ŃĪĖį | NaOHČÜŅŗ | Ę½¾łÖµ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ņ»Ī»Ķ¬Ń§ | B£® | ¶žĪ»Ķ¬Ń§ | C£® | ČżĪ»Ķ¬Ń§ | D£® | ĖÄĪ»Ķ¬Ń§ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | $\frac{b}{a}$ | B£® | $\frac{c}{a}$ | C£® | $\frac{b-2c}{a}$ | D£® | $\frac{b-c}{a}$ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·ÖÉ¢ĻµÖŠ·ÖÉ¢ÖŹĮ£×ÓµÄÖ±¾¶£ŗFe£ØOH£©3Šü×ĒŅŗ£¾Fe£ØOH£©3½ŗĢ壾FeCl3ČÜŅŗ | |
| B£® | ŃęÉ«·“Ó¦ŹĒĪļÖŹČ¼ÉÕŹ±»šŃę³ŹĻÖµÄŃÕÉ«±ä»Æ£¬ŹōÓŚ»Æѧ±ä»Æ | |
| C£® | øÖĢś·¢Éśµē»ÆѧøÆŹ“Ź±£¬øŗ¼«µÄµē¼«·“Ó¦Ź½ĪŖ Fe-3e-=Fe3+ | |
| D£® | µē½ā¾«Į¶ĶŹ±£¬Ńō¼«ÄąÖŠŗ¬ÓŠZn”¢Fe”¢Ag”¢AuµČ½šŹō |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹŅĪĀĻĀ£¬²āµĆijHAČÜŅŗÖŠc£ØHA£©=0.01mol•L-1 | |
| B£® | Ģ¼ĖįÄĘČÜŅŗÖŠµĪČė0.1mol•L-1µÄHAČÜŅŗ£¬²śÉś“óĮæĪŽÉ«ĘųĢå | |
| C£® | ŹŅĪĀĻĀ²āµĆijHAČÜŅŗµÄpH=a£¬ÓĆÕōĮóĖ®Ļ”ŹĶ100±¶²āµĆČÜŅŗpH=b£¬ĒŅb-a£¼2 | |
| D£® | ŹŅĪĀĻĀ£¬²āµĆ0.1mol•L-1HAČÜŅŗµÄpH£¾1£¬Ö¤Ć÷HAŹĒČõĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com