
£Ø9·Ö£©½«0.6 molĶĶ¶Čė100mLŅ»¶ØÅØ¶ČµÄHNO3£Ø×ćĮ棩֊£¬ÓÉÓŚHNO3µÄÅØ¶Č²»Ķ¬£¬æÉÄÜ·¢ÉśĮ½øö·“Ó¦£Ø¼ŁÉčĆæøö·“Ó¦ÖŠ²śÉśµÄĘųĢåÖ»ÓŠŅ»ÖÖ£©”£
£Ø1£©ŅŃÖŖĮ½øö·“Ó¦ÖŠØDøö·“Ó¦µÄĄė×Ó·½³ĢŹ½£Ø·“Ó¦¢Ś£©£¬Š“³öĮķØDøö·“Ó¦£Ø·“Ó¦¢Ł£©µÄĄė×Ó·½³ĢŹ½£ŗ
¢Ł____________________________________________________________________£»
¢Ś3Cu+8H++2NO3”„ ===== 3Cu2++2NO”ü+4H2O”£
£Ø2£©¼ŁÉčÖ»·¢Éś·“Ó¦¢Ł£¬²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ_____L£»¼ŁÉčÖ»·¢Éś·“Ó¦¢Ś£¬²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ________L”£
£Ø3£©Źµ¼Ź²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ22.4 L£¬Ōņ²Ī¼Ó·“Ó¦¢ŁµÄĶÓė²Ī¼Ó·“Ó¦¢ŚµÄĶµÄĪļÖŹµÄĮæÖ®±ČĪŖ___________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
”°ĒāÄÜ”±½«ŹĒĪ“Ą“×īĄķĻėµÄŠĀÄÜŌ“”£
¢ń.ŹµŃé²āµĆ£¬1gĒāĘųČ¼ÉÕÉś³ÉŅŗĢ¬Ė®Ź±·Å³ö142.9kJČČĮ棬ŌņĒāĘųČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ_______”££ØĢīŠņŗÅ£©
A.2H2£Øg£©+O2£Øg£© ![]() 2H2O£Øl£© ”÷H= £142.9kJ”¤mol”Ŗ1
2H2O£Øl£© ”÷H= £142.9kJ”¤mol”Ŗ1
B.H2£Øg£©+1/2 O2£Øg£© ![]() H2O£Øl£© ”÷H= £285.8kJ”¤mol”Ŗ1
H2O£Øl£© ”÷H= £285.8kJ”¤mol”Ŗ1
C.2H2+O2![]() 2H2O£Øl£© ”÷H= £571.6kJ”¤mol”Ŗ1
2H2O£Øl£© ”÷H= £571.6kJ”¤mol”Ŗ1
D.H2£Øg£©+1/2O2£Øg£© ![]() H2O£Øg£© ”÷H= £285.8kJ”¤mol”Ŗ1
H2O£Øg£© ”÷H= £285.8kJ”¤mol”Ŗ1
¢ņ.ij»Æѧ¼Ņøł¾Ż”°Ō×Ó¾¼Ć”±µÄĖ¼Ļė£¬Éč¼ĘĮĖČēĻĀÖʱøH2µÄ·“Ó¦²½Öč
¢ŁCaBr2+H2O![]() CaO+2HBr ¢Ś2HBr+Hg
CaO+2HBr ¢Ś2HBr+Hg![]() HgBr2+H2
HgBr2+H2
¢ŪHgBr2+_____![]() ______________ ¢Ü2HgO
______________ ¢Ü2HgO 2Hg+O2”ü
2Hg+O2ӟ
ĒėÄćøł¾Ż”°Ō×Ó¾¼Ć”±µÄĖ¼ĻėĶź³ÉÉĻŹö²½Öč¢ŪµÄ»Æѧ·½³ĢŹ½£ŗ____________”£
øł¾Ż”°ĀĢÉ«»Æѧ”±µÄĖ¼ĻėĘĄ¹ĄøĆ·½·ØÖĘH2µÄÖ÷ŅŖȱµć£ŗ______________”£
¢ó.ĄūÓĆŗĖÄÜ°ŃĖ®·Ö½āÖĘĒāĘų£¬ŹĒÄæĒ°ÕżŌŚŃŠ¾æµÄæĪĢā”£ĻĀĶ¼ŹĒĘäÖŠµÄŅ»ÖÖĮ÷³Ģ£¬ĘäÖŠÓĆĮĖ¹żĮæµÄµā”££ØĢįŹ¾£ŗ·“Ó¦¢ŚµÄ²śĪļŹĒO2”¢SO2ŗĶH2O£©

Ķź³ÉĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
·“Ó¦¢Ł__________________________£»·“Ó¦¢Ś__________________________”£
“Ė·ØÖĘČ”ĒāĘųµÄ×ī“óÓŵćŹĒ_______________________________________________”£
¢ō.ĒāĘųĶس£ÓĆÉś²śĖ®ĆŗĘųµÄ·½·ØÖʵƔ£ĘäÖŠCO£Øg£©+ H2O£Øg£© CO2£Øg£©+ H2£Øg£©; ”÷H<0”£
ŌŚ850”ꏱ£¬K=1”£
£Ø1£©ČōÉżøßĪĀ¶Čµ½950”ꏱ£¬“ļµ½Ę½ŗāŹ±K______1£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©
£Ø2£©850”ꏱ£¬ČōĻņŅ»ČŻ»żæɱäµÄĆܱÕČŻĘ÷ÖŠĶ¬Ź±³äČė 1.0 mol CO”¢3.0molH2O”¢1.0mol CO2 ŗĶ x mol H2£¬Ōņ£ŗ
¢Łµ±x=5.0Ź±£¬ÉĻŹöĘ½ŗāĻņ___________£ØĢīÕż·“Ó¦»ņÄę·“Ó¦£©·½Ļņ½ųŠŠ”£
¢ŚČōŅŖŹ¹ÉĻŹö·“Ó¦æŖŹ¼Ź±ĻņÕż·“Ó¦·½Ļņ½ųŠŠ£¬ŌņxÓ¦Āś×ćµÄĢõ¼žŹĒ__________”£
£Ø3£©ŌŚ850”ꏱ£¬ČōÉčx£½5.0molŗĶx£½6.0mol£¬ĘäĖüĪļÖŹµÄĶ¶ĮĻ²»±ä£¬µ±ÉĻŹö·“Ó¦“ļµ½Ę½ŗāŗ󣬲āµĆH2µÄĢå»ż·ÖŹż·Ö±šĪŖa£„”¢b£„£¬Ōņa_______ b£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©”£
¢õ.ĒāĘų»¹ŌŃõ»ÆĶĖłµĆµÄŗģÉ«¹ĢĢåæÉÄÜŹĒĶÓėŃõ»ÆŃĒĶµÄ»ģŗĻĪļ£¬ŅŃÖŖCu2OŌŚĖįŠŌČÜŅŗÖŠæÉ·¢Éś×ŌÉķŃõ»Æ»¹Ō·“Ó¦£¬Éś³ÉCu2+ŗĶµ„ÖŹĶ”£
£Ø1£©ĻÖÓŠ8æĖŃõ»ÆĶ±»ĒāĘų»¹Ōŗó£¬µĆµ½ŗģÉ«¹ĢĢå6.8æĖ£¬ĘäÖŠŗ¬µ„ÖŹĶÓėŃõ»ÆŃĒĶµÄĪļÖŹµÄĮæÖ®±ČŹĒ £»
£Ø2£©Čō½«6.8æĖÉĻŹö»ģŗĻĪļÓė×ćĮæµÄĻ”ĮņĖį³ä·Ö·“Ó¦ŗó¹żĀĖ£¬æɵƵ½¹ĢĢå g£»
£Ø3£©Čō½«6.8æĖÉĻŹö»ģŗĻĪļÓėŅ»¶ØĮæµÄÅØĻõĖį³ä·Ö·“Ó¦£¬
¢ŁÉś³É±ź×¼×“æöĻĀ1.568ÉżµÄĘųĢå£Ø²»æ¼ĀĒNO2µÄČܽā£¬Ņ²²»æ¼ĀĒNO2ÓėN2O4µÄ×Ŗ»Æ£©£¬ŌņøĆĘųĢåµÄ³É·ÖŹĒ £¬ĘäĪļÖŹµÄĮæÖ®±ČŹĒ £»
¢Ś°ŃµĆµ½µÄČÜŅŗŠ”ŠÄÕō·¢ÅØĖõ£¬°ŃĪö³öµÄ¾§Ģå¹żĀĖ£¬µĆ¾§Ģå23.68g”£¾·ÖĪö£¬ŌČÜŅŗÖŠµÄCu2+ÓŠ20%²ŠĮōŌŚÄøŅŗÖŠ”£ĒóĖłµĆ¾§ĢåµÄ»ÆѧŹ½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģÕć½Ź”¶«Ńō֊ѧøßČżĻĀѧʌ½×¶Ī¼ģ²ā»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
”°ĒāÄÜ”±½«ŹĒĪ“Ą“×īĄķĻėµÄŠĀÄÜŌ“”£
¢ń.ŹµŃé²āµĆ£¬1gĒāĘųČ¼ÉÕÉś³ÉŅŗĢ¬Ė®Ź±·Å³ö142.9kJČČĮ棬ŌņĒāĘųČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ_______”££ØĢīŠņŗÅ£©
A£®2H2£Øg£©+O2£Øg£©  2H2O£Øl£©”÷H= £142.9kJ”¤mol”Ŗ1 2H2O£Øl£©”÷H= £142.9kJ”¤mol”Ŗ1 |
B£®H2£Øg£©+1/2 O2£Øg£©  H2O£Øl£©”÷H= £285.8kJ”¤mol”Ŗ1 H2O£Øl£©”÷H= £285.8kJ”¤mol”Ŗ1 |
C£®2H2+O2 2H2O£Øl£©”÷H= £571.6kJ”¤mol”Ŗ1 2H2O£Øl£©”÷H= £571.6kJ”¤mol”Ŗ1 |
D£®H2£Øg£©+1/2 O2£Øg£©  H2O£Øg£© ”÷H= £285.8kJ”¤mol”Ŗ1 H2O£Øg£© ”÷H= £285.8kJ”¤mol”Ŗ1 |
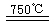 CaO+2HBr ¢Ś2HBr+Hg
CaO+2HBr ¢Ś2HBr+Hg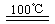 HgBr2+H2
HgBr2+H2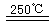 ______________ ¢Ü2HgO
______________ ¢Ü2HgO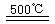 2Hg+O2”ü
2Hg+O2ӟ
 CO2£Øg£©+ H2£Øg£©; ”÷H<0”£
CO2£Øg£©+ H2£Øg£©; ”÷H<0”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğ½Ī÷Ź”°ĖŠ£øßČżĻĀѧʌĮŖæ¼Ąķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ÄæĒ°£¬”°µĶĢ¼¾¼Ć”±±øŹÜ¹Ų×¢£¬CO2µÄ²śÉś¼°ÓŠŠ§æŖ·¢ĄūÓĆ³ÉĪŖæĘѧ¼ŅŃŠ¾æµÄÖŲŅŖæĪĢā”£ŹŌŌĖÓĆĖłŃ§ÖŖŹ¶£¬½ā¾öĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŅŃÖŖij·“Ó¦µÄĘ½ŗā±ķ“ļŹ½ĪŖ£ŗ ĖüĖł¶ŌÓ¦µÄ»Æѧ·“Ó¦ĪŖ£ŗ__? ___
ĖüĖł¶ŌÓ¦µÄ»Æѧ·“Ó¦ĪŖ£ŗ__? ___
£Ø2£©”Ŗ¶ØĢõ¼žĻĀ£¬½«C(s)ŗĶH2O(g)·Ö±š¼ÓČė¼×”¢ŅŅĮ½øöĆܱÕČŻĘ÷ÖŠ£¬·¢Éś£Ø1£©ÖŠ·“Ó¦£ŗĘäĻą¹ŲŹż¾ŻČēĻĀ±ķĖłŹ¾£ŗ
ČŻĘ÷ | ČŻ»ż/L | ĪĀ¶Č/”ę | ĘšŹ¼Įæ/mol | Ę½ŗāĮæ/mol[ | “ļµ½Ę½ŗāĖłŠčŹ±¼ä/min | |
C(s) | H2O(g) | H2(g) | ||||
¼× | 2 | T1 | 2 | 4 | 3.2 | 8 |
ŅŅ | 1 | T2 | 1 | 2 | 1,2 | 3 |
¢ŁT10CŹ±£¬øĆ·“Ó¦µÄĘ½ŗā³£ŹżK=_______
¢ŚŅŅČŻĘ÷ÖŠ£¬µ±·“Ó¦½ųŠŠµ½1.5minŹ±£¬H2O(g)µÄĪļÖŹµÄĮæÅضČ_______ (ĢīŃ”Ļī×ÖÄø£©”£
A£®=0.8 mol”¤L-1??? B£®=1.4 mol”¤L-1??? C£®<1.4 mol”¤L-1??? D£®>1.4 mol”¤L-1
¢Ū±ūČŻĘ÷µÄČŻ»żĪŖ1L£¬T1”ꏱ£¬°“ĻĀĮŠÅä±Č³äČėC(s)”¢H2O(g)”¢CO2(g)ŗĶH2(g)£¬ “ļµ½Ę½?? ŗāŹ±ø÷ĘųĢåµÄĢå»ż·ÖŹżÓė¼×ČŻĘ÷ĶźČ«ĻąĶ¬µÄŹĒ_______(ĢīŃ”Ļī×ÖÄø£©”£
A.0.6 molӢ1.0 molӢ0.5 molӢ1.0 mol??
B£® 0.6 mol”¢2.0 mol”¢0 mol”¢0 mol
C.1.0 molӢ2.0 molӢ1.0 molӢ2.0 mol??????
D£® 0.25 mol”¢0.5 mol”¢0.75 mol”¢1.5 mol
£Ø3£©ŌŚŅ»¶ØĢõ¼žĻĀ£¬æĘѧ¼ŅĄūÓĆ“ÓŃĢµĄĘųÖŠ·ÖĄė³öCO2ÓėĢ«ŃōÄܵē³Ųµē½āĖ®²śÉśµÄH2ŗĻ³É¼×“¼£¬ŅŃÖŖCH3OH”¢H2µÄČ¼ÉÕČČ·Ö±šĪŖ£ŗ”÷H£½£725.5kJ/mol”¢”÷H£½£285.8kJ/mol£¬Š“³ö¹¤ŅµÉĻŅŌCO2”¢H2ŗĻ³ÉCH3OHµÄČČ»Æѧ·½³ĢŹ½£ŗ?????????????????????????????? ”£
£Ø4£©½«Č¼Ćŗ·ĻĘųÖŠµÄCO2×Ŗ»ÆĪŖ¼×Ćѵķ“Ó¦ŌĄķĪŖ£ŗ
2CO2(g£©+ 6H2(g£© CH3OCH3(g£©+ 3H2O(g)
CH3OCH3(g£©+ 3H2O(g)
ŅŃÖŖŅ»¶ØŃ¹ĒæĻĀ£¬øĆ·“Ó¦ŌŚ²»Ķ¬ĪĀ¶Č”¢²»Ķ¬Ķ¶ĮĻ±ČŹ±£¬CO2µÄ×Ŗ»ÆĀŹ¼ūĻĀ±ķ£ŗ
Ķ¶ĮĻ±Č[n(H2£©/ n(CO2)] | 500 K | 600 K | 700 K | 800 K |
1.5 | 45% | 33% | 20% | 12% |
2.0 | 60% | 43% | 28% | 15% |
3.0 | 83% | 62% | 37% | 22% |
¢ŁøĆ·“Ó¦µÄģŹ±ä”÷H?? 0£¬ģŲ±ä”÷S___0£ØĢī£¾”¢£¼»ņ£½£©”£
¢ŚÓĆ¼×ĆŃ×÷ĪŖČ¼ĮĻµē³ŲŌĮĻ£¬ŌŚ¼īŠŌ½éÖŹÖŠøƵē³Ųøŗ¼«µÄµē¼«·“Ó¦Ź½?????????????? ”£ČōŅŌ1.12 L”¤min-1£Ø±ź×¼×“æö£©µÄĖŁĀŹĻņøƵē³ŲÖŠĶØČė¼×ĆŃ£Ø·ŠµćĪŖ-24.9 ”ę£©£¬ÓĆøƵē³Ųµē½ā500 mL 2 mol”¤L-1 CuSO4ČÜŅŗ£¬Ķصē0.50 minŗó£¬ĄķĀŪÉĻæÉĪö³ö½šŹōĶ???????? g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÕć½Ź”øßČżĻĀѧʌ½×¶Ī¼ģ²ā»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
”°ĒāÄÜ”±½«ŹĒĪ“Ą“×īĄķĻėµÄŠĀÄÜŌ“”£
¢ń.ŹµŃé²āµĆ£¬1gĒāĘųČ¼ÉÕÉś³ÉŅŗĢ¬Ė®Ź±·Å³ö142.9kJČČĮ棬ŌņĒāĘųČ¼ÉÕµÄČČ»Æѧ·½³ĢŹ½ĪŖ_______”££ØĢīŠņŗÅ£©
A.2H2£Øg£©+O2£Øg£©  2H2O£Øl£©
”÷H= £142.9kJ”¤mol”Ŗ1
2H2O£Øl£©
”÷H= £142.9kJ”¤mol”Ŗ1
B.H2£Øg£©+1/2 O2£Øg£©  H2O£Øl£©
”÷H= £285.8kJ”¤mol”Ŗ1
H2O£Øl£©
”÷H= £285.8kJ”¤mol”Ŗ1
C.2H2+O2 2H2O£Øl£©
”÷H= £571.6kJ”¤mol”Ŗ1
2H2O£Øl£©
”÷H= £571.6kJ”¤mol”Ŗ1
D.H2£Øg£©+1/2
O2£Øg£©  H2O£Øg£© ”÷H= £285.8kJ”¤mol”Ŗ1
H2O£Øg£© ”÷H= £285.8kJ”¤mol”Ŗ1
¢ņ.ij»Æѧ¼Ņøł¾Ż”°Ō×Ó¾¼Ć”±µÄĖ¼Ļė£¬Éč¼ĘĮĖČēĻĀÖʱøH2µÄ·“Ó¦²½Öč
¢ŁCaBr2+H2O CaO+2HBr ¢Ś2HBr+Hg
CaO+2HBr ¢Ś2HBr+Hg HgBr2+H2
HgBr2+H2
¢ŪHgBr2+_____ ______________
¢Ü2HgO
______________
¢Ü2HgO 2Hg+O2”ü
2Hg+O2ӟ
ĒėÄćøł¾Ż”°Ō×Ó¾¼Ć”±µÄĖ¼ĻėĶź³ÉÉĻŹö²½Öč¢ŪµÄ»Æѧ·½³ĢŹ½£ŗ____________”£
øł¾Ż”°ĀĢÉ«»Æѧ”±µÄĖ¼ĻėĘĄ¹ĄøĆ·½·ØÖĘH2µÄÖ÷ŅŖȱµć£ŗ______________”£
¢ó.ĄūÓĆŗĖÄÜ°ŃĖ®·Ö½āÖĘĒāĘų£¬ŹĒÄæĒ°ÕżŌŚŃŠ¾æµÄæĪĢā”£ĻĀĶ¼ŹĒĘäÖŠµÄŅ»ÖÖĮ÷³Ģ£¬ĘäÖŠÓĆĮĖ¹żĮæµÄµā”££ØĢįŹ¾£ŗ·“Ó¦¢ŚµÄ²śĪļŹĒO2”¢SO2ŗĶH2O£©

Ķź³ÉĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
·“Ó¦¢Ł__________________________£»·“Ó¦¢Ś__________________________”£
“Ė·ØÖĘČ”ĒāĘųµÄ×ī“óÓŵćŹĒ_______________________________________________”£
¢ō.ĒāĘųĶس£ÓĆÉś²śĖ®ĆŗĘųµÄ·½·ØÖʵƔ£ĘäÖŠCO£Øg£©+ H2O£Øg£©  CO2£Øg£©+ H2£Øg£©; ”÷H<0”£
CO2£Øg£©+ H2£Øg£©; ”÷H<0”£
ŌŚ850”ꏱ£¬K=1”£
£Ø1£©ČōÉżøßĪĀ¶Čµ½950”ꏱ£¬“ļµ½Ę½ŗāŹ±K______1£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©
£Ø2£©850”ꏱ£¬ČōĻņŅ»ČŻ»żæɱäµÄĆܱÕČŻĘ÷ÖŠĶ¬Ź±³äČė 1.0 mol CO”¢3.0molH2O”¢1.0mol CO2 ŗĶ x mol H2£¬Ōņ£ŗ
¢Łµ±x=5.0Ź±£¬ÉĻŹöĘ½ŗāĻņ___________£ØĢīÕż·“Ó¦»ņÄę·“Ó¦£©·½Ļņ½ųŠŠ”£
¢ŚČōŅŖŹ¹ÉĻŹö·“Ó¦æŖŹ¼Ź±ĻņÕż·“Ó¦·½Ļņ½ųŠŠ£¬ŌņxÓ¦Āś×ćµÄĢõ¼žŹĒ__________”£
£Ø3£©ŌŚ850”ꏱ£¬ČōÉčx£½5.0 molŗĶx£½6.0mol£¬ĘäĖüĪļÖŹµÄĶ¶ĮĻ²»±ä£¬µ±ÉĻŹö·“Ó¦“ļµ½Ę½ŗāŗ󣬲āµĆH2µÄĢå»ż·ÖŹż·Ö±šĪŖa£„”¢b£„£¬Ōņa _______ b£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±£©”£
¢õ.ĒāĘų»¹ŌŃõ»ÆĶĖłµĆµÄŗģÉ«¹ĢĢåæÉÄÜŹĒĶÓėŃõ»ÆŃĒĶµÄ»ģŗĻĪļ£¬ŅŃÖŖCu2OŌŚĖįŠŌČÜŅŗÖŠæÉ·¢Éś×ŌÉķŃõ»Æ»¹Ō·“Ó¦£¬Éś³ÉCu2+ŗĶµ„ÖŹĶ”£
£Ø1£©ĻÖÓŠ8æĖŃõ»ÆĶ±»ĒāĘų»¹Ōŗó£¬µĆµ½ŗģÉ«¹ĢĢå6.8æĖ£¬ĘäÖŠŗ¬µ„ÖŹĶÓėŃõ»ÆŃĒĶµÄĪļÖŹµÄĮæÖ®±ČŹĒ £»
£Ø2£©Čō½«6.8æĖÉĻŹö»ģŗĻĪļÓė×ćĮæµÄĻ”ĮņĖį³ä·Ö·“Ó¦ŗó¹żĀĖ£¬æɵƵ½¹ĢĢå g£»
£Ø3£©Čō½«6.8æĖÉĻŹö»ģŗĻĪļÓėŅ»¶ØĮæµÄÅØĻõĖį³ä·Ö·“Ó¦£¬
¢ŁÉś³É±ź×¼×“æöĻĀ1.568ÉżµÄĘųĢå£Ø²»æ¼ĀĒNO2µÄČܽā£¬Ņ²²»æ¼ĀĒNO2ÓėN2O4µÄ×Ŗ»Æ£©£¬ŌņøĆĘųĢåµÄ³É·ÖŹĒ £¬ĘäĪļÖŹµÄĮæÖ®±ČŹĒ £»
¢Ś°ŃµĆµ½µÄČÜŅŗŠ”ŠÄÕō·¢ÅØĖõ£¬°ŃĪö³öµÄ¾§Ģå¹żĀĖ£¬µĆ¾§Ģå23.68g”£¾·ÖĪö£¬ŌČÜŅŗÖŠµÄCu2+ÓŠ20%²ŠĮōŌŚÄøŅŗÖŠ”£ĒóĖłµĆ¾§ĢåµÄ»ÆѧŹ½
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗÕć½Ź”Ä£ÄāĢā ĢāŠĶ£ŗĢīæÕĢā
 CaO+2HBr
CaO+2HBr HgBr2+H2
HgBr2+H2  ______
______  Hg+O2ӟ
Hg+O2ӟ 
 CO2(g)+H2(g) ”÷H<0 ŌŚ850”ꏱ£¬
CO2(g)+H2(g) ”÷H<0 ŌŚ850”ꏱ£¬²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com