
| ��� | �������������/mL | �������Ƶ����/mL | ��Һ��pH |
| �� | 33.00 | 0.00 | 8 |
| �� | 33.00 | x | 7 |
| �� | 33.00 | 33.00 | 6 |
���� ��1������Kw=c��H+��•c��OH-������ˮ�����ӻ���Ȼ�����ˮ�ĵ������Ϊ���ȹ����ж��¶ȴ�С��
��2������ˮ�������ӻ�����Һ��pH���������������Һ������������Ũ�ȣ�����Һ��Ϻ���Һ��pH=8�����ݸ��¶���ˮ�����ӻ���֪�����Һ��ʾ���ԣ�˵�����������ӹ����������������ݼ����x��
��3����Ӧ�����Һ��pH=6����ҺΪ���ԣ�˵������ǡ�÷�Ӧ���ݴ�д����Ӧ�����ӷ���ʽ��
��4���ȸ�������������Һ������������Ũ�ȼ��������������Ũ�ȡ�������Ũ�ȣ��ټ����ϡ��1000������Һ�б�����Ũ�ȣ�����������������Һ�У�������������ϡ��������Ũ��ֻ�����ӽ�����ʱ����Ũ�ȣ��ݴ˼��������Ũ��֮�ȣ�
��5��A��NaHSO3��Һ������˵�������������ӵ������ˮ�⣻
B��������Һ�е���غ�����жϣ�
C��������Һ�е������غ�����жϣ�
D����Һ�е����ˮ��̶Ȳ�ͬ������Ũ�Ȳ�ͬ��
��� �⣺��1�����¶���ˮ�����ӻ�Ϊ��Kw=c��H+��•c��OH-��=10-a��10-b=10-��a+b��=1��10-12��1��10-14��ˮ�ĵ���Ϊ���ȹ��̣����Ը��¶�t�棾25�棬
�ʴ�Ϊ������1��10-12��
��2������������Һ��pH=8��ˮ�������ӻ�Ϊ1��10-12������Һ������������Ũ��Ϊ��c��OH-��=10-bmol/L=$\frac{1��1{0}^{-12}}{1��1{0}^{-8}}$mol/L=10-4mol/L������b=4��
����Һ��Ϻ���Һ��pH=7������ˮ�������ӻ�Ϊ1��10-12��֪��Һ��ʾ���ԣ���Ӧ�����Һ������������Ũ��Ϊ10-5mol/L����$\frac{1��1{0}^{-4}mol/L����33-x����1{0}^{-3}L}{��33+x����1{0}^{-3}L}$=10-5mol/L����ã�x=27��
�ʴ�Ϊ��4��27��
��3�����¶��£���Һ��pH=6��˵����Һ��ʾ���ԣ���Ӧ�����ӷ���ʽΪBa2++2OH-+2H++SO42-=BaSO4��+2H2O���ʴ�Ϊ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O��
��4������������Һ������������Ũ��Ϊ��10-4mol/L��������������Ũ��Ϊ5��10-5mol/L��c��Ba2+��=c[Ba��OH��2]=5��10-5mol/L��1mL������������Һϡ�͵�1Lʱ��������Ũ��Ϊ��$\frac{5��1{0}^{-5}}{1000}$mol/L=5��10-8mol/L��������������Ũ��ֻ�����ӽ�1��10-6mol/L������ϡ�ͺ���Һ��c��Ba2+���sc��OH-��=5��10-8mol/L��1��10-6mol/L=1��20��
�ʴ�Ϊ��1��20��
��5��A������������������Һ��NaHSO3��Һ������˵�������������ӵ������ˮ�⡢��Һ������Ũ�ȴ�СΪ��c��Na+����c��HSO3-������c��SO32-����c��OH-�������Ը�����Ũ�ȹ�ϵ���ܳ�������A��ȷ��
B����Һ�д��ڵ���غ�Ϊ��c��Na+��+c��H+��=c��HRO3-��+2c��RO32-��+c��OH-������B��ȷ��
C�����ݵ���غ㣬c��Na+��+c��H+��=c��HRO3-��+2c��RO32-��+c��OH-���������غ�õ�c��Na+��=c��HRO3-��+c��RO32-��+c��H2RO3�������߽�ϵõ���c��H+��+c��H2RO3��=c��RO32-��+c��OH-������C��ȷ��
D����Һ�е����ˮ��̶Ȳ�ͬ����Һ�и�����Ũ�Ȳ�ͬ����D����
�ʴ�Ϊ��ABC��
���� ���⿼������Һ������Ũ�ȴ�С�Ƚϡ�ˮ�ĵ��뼰��Ӱ�����ء���Һ���������ҺpH�ļ����֪ʶ����Ŀ�Ѷ��еȣ������漰�������ϴ�֪ʶ��ϴ�ע�����������ʱ�Ķ����жϡ���Һ���������ҺpH�ļ��㷽������ȷ��Һ������Ũ�ȴ�С�Ƚϵķ�������4��Ϊ�״��㣬ע����ȷ����������Һϡ�ͺ�����������Ũ��Ϊ���¶��µ�����ʱ��Ũ�ȣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

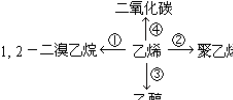
 ��CH2=CH2+H2O$\stackrel{һ������}{��}$CH3CH2OH��CH2=CH2+3O2$\stackrel{��ȼ}{��}$2CO2+2H2O��
��CH2=CH2+H2O$\stackrel{һ������}{��}$CH3CH2OH��CH2=CH2+3O2$\stackrel{��ȼ}{��}$2CO2+2H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 2C��g��+B��g����H=+150akJ/mol��
2C��g��+B��g����H=+150akJ/mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L����������Һ����ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�����ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺
ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L����������Һ����ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�����ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �²t2-t1��/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 30.6 | 5.5 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ʵ����ư��� | |
| B�� | ʵ���������Ũ�����ᾧ | |
| C�� | ʵ�������1��1��ϡ���� | |
| D�� | ʵ�������KMnO4����Һ�ζ�δ֪Ũ�ȵ�Na2SO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ��
�� ��ΪC��ͬϵ�д����������������X��һ��ͬ���칹��Ľṹ��ʽ��
��ΪC��ͬϵ�д����������������X��һ��ͬ���칹��Ľṹ��ʽ�� ��
�� ��
�� ������һ�ּ��ɣ���
������һ�ּ��ɣ��� ��
�� Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com