
£Ø10·Ö£©ÓŠ»śĪļA³£ÓĆÓŚŹ³Ę·ŠŠŅµ”£ŅŃÖŖ9.0 g AŌŚ×ćĮæO2ÖŠ³ä·ÖČ¼ÉÕ£¬½«Éś³ÉµÄ»ģŗĻĘųĢåŅĄ“ĪĶعż×ćĮæµÄÅØĮņĖįŗĶ¼īŹÆ»Ņ£¬·Ö±šŌöÖŲ5.4 gŗĶ13.2 g£¬¾¼ģŃéŹ£ÓąĘųĢåĪŖO2”£
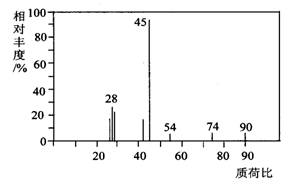
£Ø1£©A·Ö×ÓµÄÖŹĘ×Ķ¼ČēĻĀĶ¼ĖłŹ¾£¬“ÓĶ¼ÖŠæÉÖŖĘäĻą¶Ō
·Ö×ÓÖŹĮæŹĒ90£¬ŌņAµÄ·Ö×ÓŹ½ŹĒ______________________”£
£Ø2£©AÄÜÓėNaHCO3ČÜŅŗ·¢Éś·“Ó¦£¬AŅ»¶Øŗ¬ÓŠµÄ¹ŁÄÜĶÅĆū³ĘŹĒ______________”£
£Ø3£©A·Ö×ÓµÄŗĖ“Ź²ÕńĒāĘ×ÓŠ4øö·å£¬·åĆ껿֮±ČŹĒ1£ŗ1£ŗ1£ŗ3£¬ŌņAµÄ½į¹¹¼ņŹ½ŹĒ_________________________________”£
£Ø4£©0.1 mol AÓė×ćĮæNa·“Ó¦£¬ŌŚ±ź×¼×“æöĻĀ²śÉśH2µÄĢå»żŹĒ__________L”£
£Ø5£©AŌŚŅ»¶ØĢõ¼žĻĀæɾŪŗĻµĆµ½Ņ»ÖÖ¾Ūõ„£¬ÓĆÓŚÖĘŌģŹÖŹõ·ģŗĻĻߣ¬Ęä·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ___________________________”£
£Ø10·Ö£¬ĆææÕ2·Ö£©
£Ø1£©C3H6O3
£Ø2£©ōČ»ł£Ø3£© £Ø4£©2.24
£Ø4£©2.24
£Ø5£©
”¾½āĪö”æ£Ø1£©ÅØĮņĖįĪüĖ®£¬ĖłŅŌÉś³ÉµÄĖ®ŹĒ5.4g£¬ĪļÖŹµÄĮæŹĒ0.3mol”£¼īŹÆ»ŅĪüŹÕµÄŹĒCO2£¬ŌņCO2ŹĒ13.2g£¬ĪļÖŹµÄĮæŹĒ0.3mol”£ĖłŅŌ9.0gAÖŠŃõŌ×ÓµÄĪļÖŹµÄĮæŹĒ £¬Ņņ“Ė×ī¼ņŹ½ĪŖCH2O ”£ÓÖŅņĪŖĻą¶Ō·Ö×ÓÖŹĮæŹĒ90£¬ĖłŅŌ·Ö×ÓŹ½ĪŖC3H6O3 ”£
£¬Ņņ“Ė×ī¼ņŹ½ĪŖCH2O ”£ÓÖŅņĪŖĻą¶Ō·Ö×ÓÖŹĮæŹĒ90£¬ĖłŅŌ·Ö×ÓŹ½ĪŖC3H6O3 ”£
£Ø2£©AÄÜÓėNaHCO3ČÜŅŗ·¢Éś·“Ó¦£¬ŌņAŅ»¶Øŗ¬ÓŠµÄ¹ŁÄÜĶÅŹĒōČ»ł”£
£Ø3£©øł¾ŻĒāŌ×ÓµÄÖÖĄą¼°øöŹżÖ®±ČæÉÖŖ£¬½į¹¹¼ņŹ½ĪŖ ”£
ӣ
£Ø4£©AÖŠŗ¬ÓŠ1øöōĒ»łŗĶ1øöōČ»ł£¬ĖłŅŌ0.1molÄÜÉś³É0.1molĒāĘų£¬±ź×¼×“æöĻĀµÄĢå»żŹĒ2.24L”£
£Ø5£©AÖŠŗ¬ÓŠ1øöōĒ»łŗĶ1øöōČ»ł£¬ĖłŅŌÄÜĶعżõ„»Æ·“Ӧɜ³Éøß·Ö×Ó»ÆŗĻĪļ£¬·½³ĢŹ½ĪŖ




| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ÓŠ»śĪļA³£ÓĆÓŚŹ³Ę·ŠŠŅµ£®ŅŃÖŖ9.0g AŌŚ×ćĮæO2ÖŠ³ä·ÖČ¼ÉÕ£¬½«Éś³ÉµÄ»ģŗĻĘųĢåŅĄ“ĪĶعż×ćĮæµÄÅØĮņĖįŗĶ¼īŹÆ»Ņ£¬·Ö±šŌöÖŲ5.4gŗĶ13.2g£¬¾¼ģŃéŹ£ÓąĘųĢåĪŖO2£®
ÓŠ»śĪļA³£ÓĆÓŚŹ³Ę·ŠŠŅµ£®ŅŃÖŖ9.0g AŌŚ×ćĮæO2ÖŠ³ä·ÖČ¼ÉÕ£¬½«Éś³ÉµÄ»ģŗĻĘųĢåŅĄ“ĪĶعż×ćĮæµÄÅØĮņĖįŗĶ¼īŹÆ»Ņ£¬·Ö±šŌöÖŲ5.4gŗĶ13.2g£¬¾¼ģŃéŹ£ÓąĘųĢåĪŖO2£®

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ³ą·åŹŠµŚ¶žŹµŃé֊ѧø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ÓŠ»śĪļA³£ÓĆÓŚŹ³Ę·ŠŠŅµ”£ŅŃÖŖ9.0 g AŌŚ×ćĮæO2ÖŠ³ä·ÖČ¼ÉÕ£¬½«Éś³ÉµÄ»ģŗĻĘųĢåŅĄ“ĪĶعż×ćĮæµÄÅØĮņĖįŗĶ¼īŹÆ»Ņ£¬·Ö±šŌöÖŲ5.4 gŗĶ13.2 g£¬¾¼ģŃéŹ£ÓąĘųĢåĪŖO2”£
£Ø1£©A·Ö×ÓµÄÖŹĘ×Ķ¼ČēĻĀĶ¼ĖłŹ¾£¬“ÓĶ¼ÖŠæÉÖŖĘäĻą¶Ō
·Ö×ÓÖŹĮæŹĒ90£¬ŌņAµÄ·Ö×ÓŹ½ŹĒ______________________”£
£Ø2£©AÄÜÓėNaHCO3ČÜŅŗ·¢Éś·“Ó¦£¬AŅ»¶Øŗ¬ÓŠµÄ¹ŁÄÜĶÅĆū³ĘŹĒ______________”£
£Ø3£©A·Ö×ÓµÄŗĖ“Ź²ÕńĒāĘ×ÓŠ4øö·å£¬·åĆ껿֮±ČŹĒ1£ŗ1£ŗ1£ŗ3£¬ŌņAµÄ½į¹¹¼ņŹ½ŹĒ_________________________________”£
£Ø4£©0.1 mol AÓė×ćĮæNa·“Ó¦£¬ŌŚ±ź×¼×“æöĻĀ²śÉśH2µÄĢå»żŹĒ__________L”£
£Ø5£©AŌŚŅ»¶ØĢõ¼žĻĀæɾŪŗĻµĆµ½Ņ»ÖÖ¾Ūõ„£¬ÓĆÓŚÖĘŌģŹÖŹõ·ģŗĻĻߣ¬Ęä·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ___________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÉĀĪ÷Ź”įŖɽĻŲø߶žµŚ¶žŃ§ĘŚĘŚÄ©ÖŹĮæ¼ģ²ā»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ÓŠ»śĪļA³£ÓĆÓŚŹ³Ę·ŠŠŅµ”£ŅŃÖŖ9.0 g AŌŚ×ćĮæO2ÖŠ³ä·ÖČ¼ÉÕ£¬»ģŗĻĘųĢåŅĄ“ĪĶعż×ćĮæµÄÅØĮņĖįŗĶ¼īŹÆ»Ņ£¬·Ö±šŌöÖŲ5.4 gŗĶ13.2 g£¬¾¼ģŃéŹ£ÓąĘųĢåĪŖO2”£
£Ø1£©A·Ö×ÓµÄÖŹĘ×Ķ¼ČēĻĀĶ¼ĖłŹ¾£¬“ÓĶ¼ÖŠæÉÖŖĘäĻą¶Ō·Ö×ÓÖŹĮæŹĒ90£¬ŌņAµÄ·Ö×ÓŹ½ŹĒ_______________”£
£Ø2£©AÄÜÓėNaHCO3ČÜŅŗ·¢Éś·“Ó¦£¬AŅ»¶Øŗ¬ÓŠµÄ¹ŁÄÜĶÅĆū³ĘŹĒ__________________”£
£Ø3£©A·Ö×ÓµÄŗĖ“Ź²ÕńĒāĘ×ÓŠ4øö·å£¬·åĆ껿֮±ČŹĒ1£ŗ1£ŗ1£ŗ3£¬ŌņA µÄ½į¹¹¼ņŹ½ŹĒ_________________________________”£
£Ø4£©0.1 mol AÓė×ćĮæNa·“Ó¦£¬ŌŚ±ź×¼×“æöĻĀ²śÉśH2µÄĢå»żŹĒ__________L”£
£Ø5£©AŌŚŅ»¶ØĢõ¼žĻĀæɾŪŗĻµĆµ½Ņ»ÖÖ¾Ūõ„£¬ÓĆÓŚÖĘŌģŹÖŹõ·ģŗĻĻߣ¬Ęä·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģÉĀĪ÷Ź”įŖɽĻŲø߶žµŚ¶žŃ§ĘŚĘŚÄ©ÖŹĮæ¼ģ²ā»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ÓŠ»śĪļA³£ÓĆÓŚŹ³Ę·ŠŠŅµ”£ŅŃÖŖ9.0 g AŌŚ×ćĮæO2ÖŠ³ä·ÖČ¼ÉÕ£¬»ģŗĻĘųĢåŅĄ“ĪĶعż×ćĮæµÄÅØĮņĖįŗĶ¼īŹÆ»Ņ£¬·Ö±šŌöÖŲ5.4 gŗĶ13.2 g£¬¾¼ģŃéŹ£ÓąĘųĢåĪŖO2”£
£Ø1£©A·Ö×ÓµÄÖŹĘ×Ķ¼ČēĻĀĶ¼ĖłŹ¾£¬“ÓĶ¼ÖŠæÉÖŖĘäĻą¶Ō·Ö×ÓÖŹĮæŹĒ90£¬ŌņAµÄ·Ö×ÓŹ½ŹĒ_______________”£

£Ø2£©AÄÜÓėNaHCO3ČÜŅŗ·¢Éś·“Ó¦£¬AŅ»¶Øŗ¬ÓŠµÄ¹ŁÄÜĶÅĆū³ĘŹĒ__________________”£
£Ø3£©A·Ö×ÓµÄŗĖ“Ź²ÕńĒāĘ×ÓŠ4øö·å£¬·åĆ껿֮±ČŹĒ1£ŗ1£ŗ1£ŗ3£¬ŌņA µÄ½į¹¹¼ņŹ½ŹĒ_________________________________”£
£Ø4£©0.1 mol AÓė×ćĮæNa·“Ó¦£¬ŌŚ±ź×¼×“æöĻĀ²śÉśH2µÄĢå»żŹĒ__________L”£
£Ø5£©AŌŚŅ»¶ØĢõ¼žĻĀæɾŪŗĻµĆµ½Ņ»ÖÖ¾Ūõ„£¬ÓĆÓŚÖĘŌģŹÖŹõ·ģŗĻĻߣ¬Ęä·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģ±±¾©Ź¦“óø½ÖŠø߶žÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©ÓŠ»śĪļA³£ÓĆÓŚŹ³Ę·ŠŠŅµ”£ŅŃÖŖ9.0gAŌŚ×ćĮæO2ÖŠ³ä·ÖČ¼ÉÕ£¬»ģŗĻĘųĢåŅĄ“ĪĶعż×ćĮæµÄÅØĮņĖįŗĶ¼īŹÆ»Ņ£¬·Ö±šŌöÖŲ5.4gŗĶ13.2g£¬¾¼ģŃéŹ£ÓąĘųĢåĪŖO2”£
£Ø1£©A·Ö×ÓµÄÖŹĘ×Ķ¼ČēĻĀĶ¼ĖłŹ¾£¬“ÓĶ¼ÖŠæÉÖŖĘäĻą¶Ō·Ö×ÓÖŹĮæŹĒ90£¬ŌņAµÄ·Ö×ÓŹ½ŹĒ______________”£
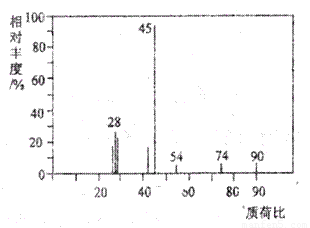

£Ø2£©AÄÜÓėNaHCO3ČÜŅŗ·¢Éś·“Ó¦£¬AŅ»¶Øŗ¬ÓŠµÄ¹ŁÄÜĶÅĆū³ĘŹĒ_____________”£
£Ø3£©A·Ö×ÓµÄŗĖ“Ź²ÕńĒāĘ×ÓŠ4øö·ę£¬·åĆ껿֮±ČŹĒ1:1:1:3£¬ŌņAµÄ½į¹¹¼ņŹ½ŹĒ_______________”£
£Ø4£©0.1mol AÓė×ćĮæNa·“Ó¦£¬ŌŚ±ź×¼×“æöĻĀ²śÉśH2µÄĢå»żŹĒ__________________L”£
£Ø5£©AŌŚŅ»¶ØĢõ¼žĻĀæɾŪŗĻµĆµ½Ņ»ÖÖ¾Ūõ„£¬ÓĆÓŚÖĘŌģŹÖŹõ·ģŗĻĻߣ¬Ęä·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ___________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com