
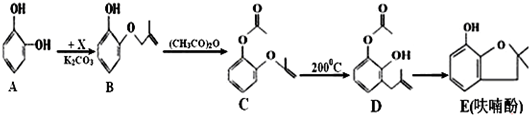
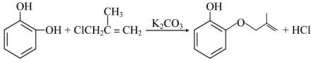 ��
��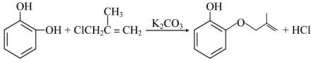 ��
�� ����5�֣�
����5�֣� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ڷ�����ѧ�г���Na2C2O4������Ϊ�����ʲⶨKMnO4��Һ��Ũ�ȣ���H2SO4��Һ�У���Ӧ���£�2MnO
�ڷ�����ѧ�г���Na2C2O4������Ϊ�����ʲⶨKMnO4��Һ��Ũ�ȣ���H2SO4��Һ�У���Ӧ���£�2MnO- 4 |
2- 4 |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ij��Һ���������ܲ���ʹ����ʯ��ˮ����ǵ����壬�����Һ��һ������HCO3-��CO32- |
| B������[KAl��SO4��2?12H2O]��ˮ�����γ�Al��OH��3���壬��������ˮ�� |
| C�����Ʊ�Fe��OH��3���壬��ʢ�з�ˮ���ձ��еμ�FeCl3������Һ����ʱ����� |
| D����SO2ͨ��Ʒ����Һ����Һ��ɫ����Ȼָ�ԭɫ����SO2ͨ����ˮ����ˮ��ɫ�����Ҳ�ָܻ�ԭɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��4 | B��5 | C��7 | D��8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
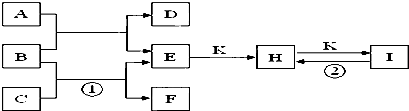
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Ʒ������� |
| B�����촶�� |
| C�������Ͻ��Ŵ� |
| D���������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
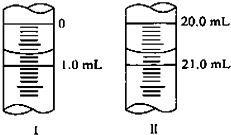
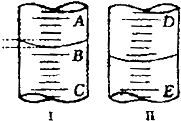 ����ͼ����1��ͼ���ʾ10mL��Ͳ��Һ���λ�ã�A��B��B��C�̶����1mL������̶�AΪ5����Ͳ��Һ�������
����ͼ����1��ͼ���ʾ10mL��Ͳ��Һ���λ�ã�A��B��B��C�̶����1mL������̶�AΪ5����Ͳ��Һ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | X | Y | Z |
| ��ʼŨ��/mol?L-1 | 0.1 | 0.2 | 0 |
| ƽ��Ũ��mol?L-1 | 0.05 | 0.05 | 0.1 |
| A����Ӧ�ﵽƽ��ʱ��X��ת����Ϊ50% |
| B����Ӧ�ɱ�ʾΪX+3Y?2Z |
| C������ѹǿʹƽ��������Z�ķ����ƶ� |
| D�������������ʹƽ�������ƶ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com