
ĻÖÓŠļ§ŃĪѳʷA£¬ŹĒ(NH4)2SO4”¢NH4HSO4µÄ»ģŗĻĪļ”£ĪŖČ·¶ØAÖŠø÷³É·ÖµÄŗ¬Įæ£¬Ä³ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§Č”ĮĖŹż·ŻĻąĶ¬ÖŹĮæµÄѳʷAČÜÓŚĖ®£¬Č»ŗó·Ö±š¼ÓČė²»Ķ¬Ģå»żµÄ1 mol/LµÄNaOHČÜŅŗ£¬Ė®Ō”¼ÓČČÖĮĘųĢåČ«²æŅŻ³ö(“ĖĪĀ¶ČĻĀ£¬ļ§ŃĪ²»·Ö½ā)”£øĆĘųĢåøÉŌļŗóÓĆ×ćĮæµÄÅØĮņĖįĶźČ«ĪüŹÕ”£ÅØĮņĖįŌöÖŲµÄÖŹĮæÓė¼ÓČėNaOHČÜŅŗµÄĢå»żµÄ¹ŲĻµČēĶ¼ĖłŹ¾”£·ÖĪöøĆĶ¼Ļó²¢»Ų“šĻĀĮŠĪŹĢā£ŗ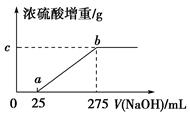
(1)Š“³öab¶ĪÉę¼°µÄĄė×Ó·½³ĢŹ½£ŗ_____________________________”£
(2)cµć¶ŌÓ¦µÄŹżÖµŹĒ________£»ŃłĘ·AÖŠ(NH4)2SO4”¢NH4HSO4µÄĪļÖŹµÄĮæÖ®±ČĪŖ________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŚ±ź×¼×“æöĻĀ15 g COÓėCO2µÄ»ģŗĻĘųĢ壬Ģå»żĪŖ11.2 L”£Ōņ£ŗ
(1)»ģŗĻĘųĢåµÄĆܶȏĒ ”£
(2)»ģŗĻĘųĢåµÄĘ½¾łÄ¦¶ūÖŹĮæŹĒ ”£
(3)CO2ŗĶCOµÄĢå»żÖ®±ČŹĒ ”£
(4)COµÄĢå»ż·ÖŹżŹĒ ”£
(5)CO2ŗĶCOµÄÖŹĮæÖ®±ČŹĒ ”£
(6)COµÄÖŹĮæ·ÖŹżŹĒ ”£
(7)»ģŗĻĘųĢåÖŠĖłŗ¬ŃõŌ×ÓµÄĪļÖŹµÄĮæŹĒ ”£
(8)»ģŗĻĘųĢåÖŠĖłŗ¬Ģ¼Ō×ÓµÄĪļÖŹµÄĮæŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
36 g H2OÓė80 g D2OµÄĪļÖŹµÄĮæÖ®±ČŹĒ______£¬·Ö×ÓÖŠĖłŗ¬ÖŹ×ÓŹżÖ®±ČŹĒ__________£¬Ėłŗ¬ÖŠ×ÓŹżÖ®±ČŹĒ________£¬ĖüĆĒ·Ö±šÓėNa·“Ó¦Ź±£¬Ėł·Å³öĘųĢåĢå»żÖ®±Č£ØĶ¬Ģõ¼ž£©ŹĒ______£¬ÖŹĮæÖ®±ČŹĒ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÅäÖĘ500 mL 0.5 mol/LµÄNaOHČÜŅŗ,ŹŌ»Ų“šĻĀĮŠĪŹĢā:
(1)¼ĘĖć:ŠčŅŖNaOH¹ĢĢåµÄÖŹĮæĪŖ ”£
(2)ÅäÖĘ·½·Ø:Éč¼ĘĪåøö²Ł×÷²½Öč”£
¢ŁĻņŹ¢ÓŠNaOH¹ĢĢåµÄÉÕ±ÖŠ¼ÓČė200 mLÕōĮóĖ®Ź¹ĘäČܽā,²¢ĄäČ“ÖĮŹŅĪĀ;
¢Ś¼ĢŠųĶłČŻĮæĘæÖŠ¼ÓÕōĮóĖ®ÖĮŅŗĆę½Ó½üæĢ¶ČĻß1”«2 cm;
¢Ū½«NaOHČÜŅŗŃŲ²£Į§°ō×¢Čė500 mLČŻĮæĘæÖŠ;
¢ÜÓĆÉŁĮæµÄÕōĮóĖ®Ļ“µÓÉÕ±ŗĶ²£Į§°ō2”«3“Ī,Č»ŗó½«Ļ“µÓŅŗŅĘČėČŻĮæĘæ;
¢ŻøÄÓĆ½ŗĶ·µĪ¹Ü¼ÓÕōĮóĖ®ÖĮæĢ¶ČĻß,¼ÓøĒŅ”ŌČ”£
ŹŌ½«²Ł×÷²½ÖčÕżČ·ÅÅŠņ ”£
(3)Ä³Ń§ÉśŹµ¼ŹÅäÖĘNaOHČÜŅŗµÄÅضČĪŖ0.48 mol/L,ŌŅņæÉÄÜŹĒ ”£
| A£®Ź¹ÓĆĀĖÖ½³ĘĮæĒāŃõ»ÆÄĘ¹ĢĢå |
| B£®ČŻĮæĘæÖŠŌĄ““ęŌŚÉŁĮæÕōĮóĖ® |
| C£®ČܽāŗóµÄÉÕ±Ī“¾¶ą“ĪĻ“µÓ |
| D£®½ŗĶ·µĪ¹Ü¼ÓĖ®¶ØČŻŹ±ŃöŹÓæĢ¶ČĻß |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶žĮņ»ÆŃĒĢśŹĒLi/FeS2µē³ŲµÄÕż¼«»īŠŌĪļÖŹ£¬æÉÓĆĖ®ČČ·ØŗĻ³É”£FeSO4”¢Na2S2O3”¢S¼°H2OŌŚ200 ”ęĮ¬Šų·“Ó¦24 h£¬ĖÄÖÖĪļÖŹŅŌµČĪļÖŹµÄĮæ·“Ó¦£¬ŌŁŅĄ“ĪÓĆCS2”¢H2OĻ“µÓ”¢øÉŌļ¼°¾§»ÆŗóµĆµ½”£
(1)ŗĻ³ÉFeS2µÄĄė×Ó·½³ĢŹ½ĪŖ_____________________________________”£
(2)ÓĆĖ®Ļ“µÓŹ±£¬ČēŗĪÖ¤Ć÷SO42”ŖŅŃ³ż¾”£æ________________________________________”£
(3)ŅŃÖŖ1.20 g FeS2ŌŚO2ÖŠĶźČ«Č¼ÉÕÉś³ÉFe2O3ŗĶSO2ĘųĢå·Å³ö8.52 kJČČĮ棬FeS2Č¼ÉÕ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ__________________________”£
(4)Č”ÉĻŹöÖʵƵÄÕż¼«²ÄĮĻ1.120 0 g(¼Ł¶ØÖ»ŗ¬FeSŅ»ÖÖŌÓÖŹ)£¬ŌŚ×ćĮæµÄŃõĘųĮ÷ÖŠ³ä·Ö¼ÓČČ£¬×īŗóµĆ0.800 0 gŗģ×ŲÉ«¹ĢĢ壬ŹŌ¼ĘĖćøĆÕż¼«²ÄĮĻÖŠFeS2µÄÖŹĮæ·ÖŹż(Š“³ö¼ĘĖć¹ż³Ģ)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ĪŖŹµŃéŹŅijŃĪĖįŹŌ¼ĮĘæ±źĒ©ÉĻµÄÓŠ¹ŲŹż¾Ż£¬ŹŌøł¾Ż±źĒ©ÉĻµÄÓŠ¹ŲŹż¾Ż»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©øĆÅØŃĪĖįÖŠHClµÄĪļÖŹµÄĮæÅضČĪŖ mol?L-1”£
£Ø2£©Č”ÓĆČĪŅāĢå»żµÄøĆŃĪĖįČÜŅŗŹ±£¬ĻĀĮŠĪļĄķĮæÖŠ²»ĖęĖłČ”Ģå»żµÄ¶ąÉŁ¶ų±ä»ÆµÄŹĒ ”£
A£®ČÜŅŗÖŠHClµÄĪļÖŹµÄĮæ B£®ČÜŅŗµÄÅضČ
C£®ČÜŅŗÖŠCl-µÄŹżÄæ D£®ČÜŅŗµÄĆܶČ
£Ø3£©Ä³Ń§ÉśÓūÓĆÉĻŹöÅØŃĪĖįŗĶÕōĮóĖ®ÅäÖĘ480 mLĪļÖŹµÄĮæÅضČĪŖ0.400 mol?L-1µÄĻ”ŃĪĖį”£
¢ŁČŻĮæĘæÉĻŠč±źÓŠŅŌĻĀĪåĻīÖŠµÄ ”£
A£®ĪĀ¶Č B£®ÅØ¶Č C£®ČŻĮæ D£®Ń¹Ēæ E£®æĢ¶ČĻß
¢Ś½«ĻĀĮŠ²Ł×÷ĢīŠ“ĶźÕū£¬²¢ÅÅĮŠĘäÕżČ·µÄ²Ł×÷Ė³Šņ £Ø×ÖÄø±ķŹ¾£¬Ćæøö×ÖÄøÖ»ÄÜÓĆŅ»“Ī£©£»
A£®ÓĆ30mLĖ®Ļ“µÓÉÕ±2”«3“Ī£¬Ļ“µÓŅŗ¾ł×¢ČėČŻĮæĘ棬Õńµ“
B£®ÓĆĮæĶ²×¼Č·ĮæČ”ÅØŃĪĖį mL£¬×¢ČėÉÕ±ÖŠ£¬¼ÓČėÉŁĮæĖ®£ØŌ¼30mL£©£¬ÓĆ²£Į§°ōĀżĀż½Į°č£¬Ź¹Ęä»ģŗĻ¾łŌČ
C£®½«ŅŃĄäČ“µÄŃĪĖįŃŲ²£Į§±×¢Čė ÖŠ
D£®½«ČŻĮæĘæøĒ½ō£¬µßµ¹Ņ”ŌČ
E£®øÄÓĆ ¼ÓĖ®£¬Ź¹ČÜŅŗ°¼ŅŗĆęĒ”ŗĆÓėæĢ¶ČĻßĻąĒŠ
F£®¼ĢŠųĶłČŻĮæĘæÄŚŠ”ŠÄ¼ÓĖ®£¬Ö±µ½ŅŗĆęÖĮæĢ¶ČĻßĻĀ “¦”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
øł¾ŻĻĀĮŠø÷ĢāĖłøų³öµÄŹż¾Ż£¬æÉ·Ö±šĒó³öĘä”°ČÜÖŹµÄÖŹĮæ·ÖŹż”±»ņ”°ČÜÖŹµÄĪļÖŹµÄĮæÅØ¶Č”±£¬ŹŌÅŠ¶Ļ²¢Ēó½ā”£
£Ø1£©ÉčNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£ŹżµÄŹżÖµ£¬ČōijĒāŃõ»ÆÄĘČÜŅŗV LÖŠŗ¬ÓŠNøöOH-£¬ŌņæÉĒó³ö“ĖČÜŅŗÖŠ______ĪŖ______”£
£Ø2£©ŅŃÖŖijĒāŃõ»ÆÄĘČÜŅŗÖŠNa£«ÓėH2OµÄøöŹżÖ®±ČĪŖ1”Ća£¬ŌņæÉĒó³ö“ĖČÜŅŗÖŠ______ĪŖ______”£
£Ø3£©ŅŃÖŖ±ź×¼×“æöĻĀ1Ģå»żĖ®ÄÜČܽā500Ģå»żµÄĀČ»ÆĒā£¬ŌņæÉĒó³ö±ź×¼×“æöĻĀĀČ»ÆĒā±„ŗĶČÜŅŗÖŠ______ĪŖ______”£
£Ø4£©ŅŃÖŖ½«100 mLĀČ»ÆĀĮµÄĖ®ČÜŅŗ¼ÓČČÕōøÉ×ĘÉÕ£¬æɵƵ½°×É«¹ĢĢåb g£¬ŌņæÉĒó³öŌĀČ»ÆĀĮČÜŅŗÖŠ______ĪŖ______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijŠ”×éÓĆĶŠ¼ÖĘČ”ĮņĖįĶČÜŅŗ”£½«ĶŠ¼·ÅČėŅ»¶ØÅØ¶ČµÄĮņĖįÖŠ£¬¼ÓČČ²¢²»¶Ļ¹ÄČėæÕĘųĘä·“Ó¦ŌĄķĪŖ2Cu£«O2£«2H2SO4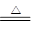 2CuSO4£«2H2O”£ĻÖÓū½«6.4 gĶĶźČ«Čܽā£¬¼ÓĖ®ŗóµĆ200 mLČÜŅŗ”£¼ĘĖć£ŗ
2CuSO4£«2H2O”£ĻÖÓū½«6.4 gĶĶźČ«Čܽā£¬¼ÓĖ®ŗóµĆ200 mLČÜŅŗ”£¼ĘĖć£ŗ
£Ø1£©²Ī¼Ó·“Ó¦µÄŃõĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żŹĒ ”£
£Ø2£©ĖłµĆĮņĖįĶČÜŅŗµÄĪļÖŹµÄĮæÅØ¶ČŹĒ ”£
£Ø3£©Č”100 mLÉĻŹöĮņĖįĶČÜŅŗ£¬¼ÓĖ®Ļ”ŹĶÖĮ0.1 mol/L£¬Ļ”ŹĶŗóĮņĖįĶČÜŅŗµÄĢå»żŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŌŚŹµŃéŹŅÖŠ£¬µŖŃõ»ÆĪļ·ĻĘų£ØÖ÷ŅŖ³É·ÖNO2ŗĶNO£©æÉŅŌÓĆNaOHČÜŅŗĄ“ĪüŹÕ£¬³żČ„ÕāŠ©·ĻĘų£¬ĘäÖ÷ŅŖ·“Ó¦ĪŖ£ŗ2NO2 +2 NaOH ”śNaNO2 + NaNO3 + H2O NO + NO2 + 2NaOH ”ś 2NaNO2 + H2O
£Ø1£©2molNOŗĶ2.4molNO2»ģŗĻĘųĢåĶØČėNaOHČÜŅŗ±»ĶźČ«ĪüŹÕŹ±£¬Éś³ÉµÄNaNO2
ŹĒ_______mol£»Éś³ÉµÄNaNO3ŹĒ_______mol ”£
£Ø2£©NOŗĶNO2µÄ»ģŗĻĘųĢåµÄ×é³ÉæɱķŹ¾ĪŖNOX £¬øĆ»ģŗĻĘųĢåĶØČėNaOHČÜŅŗ±»
ĶźČ«ĪüŹÕŹ±£¬xµÄÖµæÉŅŌĪŖ £ØĢī±ąŗÅ£©”£
a£®1.1 b£®1.2 c£®1.5 d£®1.8
£Ø3£©ČōÓĆ“æ¼īČÜŅŗ“¦ĄķµŖŃõ»ÆĪļ·ĻĘų£¬·“Ó¦ÓėÉĻŹöĄąĖĘ£¬Ķ¬Ź±·Å³öCO2”£
ĒėŠ“³ö“æ¼īČÜŅŗĪüŹÕNO2µÄ»Æѧ·½³ĢŹ½£ŗ____________________
£Ø4£©ĻÖÓŠ±ź×¼×“æöĻĀaÉżNO2£ØĘäÖŠN2O4Ģå»ż·ÖŹżĪŖ20%£©ŗĶbÉżNOµÄ»ģŗĻĘųĒ”ŗƱ»200mL Na2CO3ČÜŅŗĶźČ«ĪüŹÕ£¬Ōņa”¢bÓ¦Āś×ćµÄ¹ŲĻµĪŖ£ŗ £»øĆNa2CO3ČÜŅŗµÄĪļÖŹµÄĮæÅضČĪŖ ________mol/L£ØÓĆŗ¬a”¢bµÄ“śŹżŹ½±ķŹ¾£©”£
£Ø5£©ČōŌŚ±ź×¼×“æöĻĀ£¬2.016 LµŖŃõ»ÆĪļµÄ»ģŗĻĘųŗĶ0.896LO2Óė1mol/LNa2CO3ČÜŅŗ50mLĒ”ŗĆ·“Ӧɜ³ÉNaNO3£¬Ōņ»ģŗĶĘųĢåÖŠN2O4ÓėNO2µÄĢå»ż±ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com