
 CaCl2+2H2O+2NH3����
CaCl2+2H2O+2NH3���� CaCl2+2H2O+2NH3����
CaCl2+2H2O+2NH3����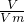 =
=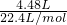 =0.2mol���õ���0.05L��Һ�����ʵ���Ũ����c=
=0.2mol���õ���0.05L��Һ�����ʵ���Ũ����c= =
= =4mol/L��
=4mol/L�� L��l mol/L=0.005mol��a=5��
L��l mol/L=0.005mol��a=5��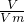 ���㰱�������ʵ���������c=
���㰱�������ʵ���������c= ����������Һ�����ʵ���Ũ�ȣ�
����������Һ�����ʵ���Ũ�ȣ�

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�
��1��ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ�� ��
��2����4.48L����״��������ͨ��ˮ�еõ�0.05L��Һ��������Һ�����ʵ���Ũ���� ��
��3������100mL AlCl3��MgSO4�Ļ����Һ���ֳ����ȷݡ�
��������һ���м���10mL 4mol/L�İ�ˮ��ǡ����ȫ��Ӧ������AlCl3�백ˮ��Ӧ�����ӷ���ʽ�� ����������l mol/L NaOH��Һ��10mLʱ���������ټ��٣��������ٵ����ӷ���ʽ�� �����ٵij��������ʵ����� ��
������һ���м���a mL 1mol/LBaCl2��Һ��ʹSO42��������ȫ��a= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�걱��ʦ���и�һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��12�֣�
��1��ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ�� ��
��2����4.48L����״��������ͨ��ˮ�еõ�0.05L��Һ��������Һ�����ʵ���Ũ���� ��
��3������100mL AlCl3��MgSO4�Ļ����Һ���ֳ����ȷݡ�
��������һ���м���10mL 4mol/L�İ�ˮ��ǡ����ȫ��Ӧ������AlCl3�백ˮ��Ӧ�����ӷ���ʽ�� ����������l mol/L NaOH��Һ��10mLʱ���������ټ��٣��������ٵ����ӷ���ʽ�� �����ٵij��������ʵ����� ��
������һ���м���a mL 1mol/LBaCl2��Һ��ʹSO42��������ȫ��a= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�챱��ʦ���и�һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��12�֣�
��1��ʵ�������Ȼ�粒�����ȡ�����Ļ�ѧ����ʽ�� ��
��2����4.48L����״��������ͨ��ˮ�еõ�0.05L��Һ��������Һ�����ʵ���Ũ���� ��
��3������100mL AlCl3��MgSO4�Ļ����Һ���ֳ����ȷݡ�
��������һ���м���10mL 4mol/L�İ�ˮ��ǡ����ȫ��Ӧ������AlCl3�백ˮ��Ӧ�����ӷ���ʽ�� ����������l mol/L NaOH��Һ��10mLʱ���������ټ��٣��������ٵ����ӷ���ʽ�� �����ٵij��������ʵ����� ��
������һ���м���a mL 1mol/LBaCl2��Һ��ʹSO42��������ȫ��a= ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com