
��ͼΪ���ֶ�����Ԫ�ػ��ϼ���ԭ�������Ĺ�ϵͼ����ش��������⡣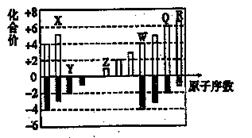
��1��Ԫ��Qλ�����ڱ��е� ����______�塣
��2����X��Z��Q��R����Ԫ�ص��⻯���1mol��ˮ��Ϻ���õ�l L��Һ������ˮ��Һ������ǿ���⻯��ĵ���ʽ�� ��
��3��Z������������Ӧ��ˮ������W�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ ��
��4��ˮ���̺������꣬����ʹ��ߺ�����Ⱦɫ��ʹˮ������ζ��RY2����������ȥˮ�г����Mn2+������һ�ֺ�ɫ������ͬʱ�������13��50 g RY2ʱ����ת����1 mol���ӡ���Ӧ�����ӷ���ʽ��____��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������γɵĸ�����ʯ��(CaSO4��2H2O)ת��Ϊ����ط��Ϻ��Ȼ���ˮ���ﴢ�Ȳ��ϣ����۴Ӿ���Ч�桢��Դ�ۺ����û��Ǵӻ��������Ƕȿ���������Ҫ���塣������ʯ��ת��Ϊ����غ��Ȼ��ƵĹ�������ʾ��ͼ��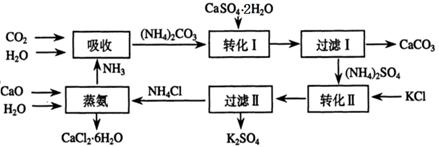
��1�������������õ�ԭ�ϳ�CaSO4��2H2O��KCl�⣬����Ҫ______________��ԭ�ϡ�
��2��ʯ������Һ�м���̼�����Һ������Ӧ�����ӷ���ʽΪ_____________��
��3�����ˢ�������ù����У���CaCO3����� ���ѧʽ�������ʣ��ù������������ˮ���ԭ�ϡ�
��4��������ˢ�������Һ�к���CO32���ķ�����_____________________________��
��5���Ȼ��ƽᾧˮ����(CaCl2��6H2O)��Ŀǰ���õ������Ȳ��ϣ�ѡ���������___��
a���۵�ϵͣ�29���ۻ��� b���ܵ��� c�������� d����
��6����������������������ɫ��ѧ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ڶ���������Ԫ���У�
��1��������Ԫ����ɵĻ�����������࣬�����������ĵ���ʽΪ ��
��2��Ԫ��D���⻯����D������������Ӧˮ���ﻯ�ϵõ��������ǣ��ѧʽ��__________��D���⻯���ˮ��Һ������pH_____7���������������������������D������������Ӧˮ�����Ũ��Һ���Թ�������ͭƬ��Ӧ����ʼ������ɫ���壬һ��ʱ����������ɫ���壬д��������ɫ��������ӷ���
ʽ ��
��3��Ԫ��R��Ԫ��E��ԭ������֮��Ϊ2��1���䵥��ֱ�ӷ�Ӧ��õ��Ļ�������Ԫ��������Ϊ1��1���û�����Ļ�ѧʽΪ________���û�����_______����ܡ����ܡ���ʹƷ����Һ��ɫ������2 mol RԪ�ص�����������Ӧˮ�����Ũ��Һ��������ͭƬ��Ӧ�������������ڱ�״���µ����һ���� 22.4 L������ڡ�����С�ڡ����ڡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ס��ҡ���������������֮���������ת����ϵ��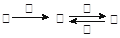
��ش��������⣺
��1������Ϊ���Բ�������ת��Ϊ�ҵ����ӷ���ʽΪ ��
��2������Ϊ�γ��������Ҫ���ʣ���Ļ�ѧʽ ���������ȵ�NaOH��Һ��Ӧ��������Ԫ�ص����̬Ϊ+4��д���÷�Ӧ�����ӷ���ʽΪ��
��
��3�������к���Ŀǰ����ʹ����㷺�Ľ���Ԫ�أ�����ת���ɱ�Ϊ���Ϸ�Ӧ������Һ���������յõ��������� ����ȥ����Һ�������ҵķ����ǣ� ���û�ѧ����ʽ��ʾ������μ�������Һ�еı��������ʵ�鷽�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ԫ��X��Y��Zԭ���������ε�����X��Y��Z������������֮��Ϊ13��Y�����������н�������ǿ�ģ�X��Z��ͬһ���塣
��1��ZԪ�������ڱ��е�λ���� ��
��2��Y2ZX3��Һ�� �ԣ���֤������Һ�д���ˮ��ƽ�����ʵ�� ������ţ���
a�������̪��Һ��죬�ټ���ϡH2SO4��ɫ��ȥ
b�������̪��Һ��죬�ټ�����ˮ��ɫ��ȥ
c�������̪��Һ��죬�ټ���BaCl2��Һ���������Һ�ɫ��ȥ
��3��������Cu2X��Cu2Z�ɷ�������ת��������D����ά��ˮ������ղ����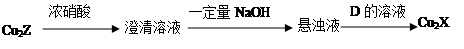
�� D�Ļ�ѧʽ�� ��
�� Cu2X��Ũ���ᷴӦ�к���ɫ�������ɣ���ѧ��Ӧ����ʽ�� ��
��4��ij�¶��£���һ��5L���ܱ������г���0.2 mol ZX2��0.1 mol X2��20 s��ﵽƽ�⣬��������к���0.18 mol ZX3������X2��ʾ�÷�Ӧ��ƽ��������v(X2) = �����¶��¸÷�Ӧ�Ļ�ѧƽ�ⳣ��K= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й��������ʵıȽ���ȷ����
��ͬ����Ԫ�صĵ��ʴ��ϵ��£��ǽ����Լ������۵�����
��Ԫ�ص���������ϼ�����ֵ�ϵ��������ڵ�������
��ͬ��������Ԫ�ص�ԭ�Ӱ뾶ԽС��Խ��ʧȥ����
��Ԫ�صķǽ�����Խǿ��������̬�⻯��ˮ��Һ������Խǿ
�ݻ�ԭ�ԣ�S2����Se2����Br����Cl��
�����ԣ�HClO4��H2SO4��H3PO4��H2SiO3
| A���٢� | B���ڢ� | C���ۢ� | D���ݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪����Ԫ�ص�ԭ�Ӱ뾶��
| Ԫ�� | N | S | O | Si |
| ԭ�Ӱ뾶/10-10m | 0.75 | 1.02 | 0.74 | 1.17 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��˵��M�ķǽ����Ա�Nǿ����ʵ��
| A��M����̬�⻯���N����̬�⻯���ȶ� |
| B��HxM�����Ա�HyN������ǿ |
| C������M���۷е�ȵ���N���ܷе�� |
| D��M�ܽ�N����������Һ���û����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ҹ����±����ĸ߳������У��裨Tl��������֮һ����֪λ�ڵ������ڵڢ�A�壬�������˵�������ܴ������ �� ��
| A��������ɫ���� | B��Tl��OH��3 ������ |
| C����ϡ���ᷴӦ���������� | D����Ľ�����ǿ�����Ľ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com