
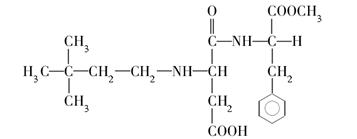
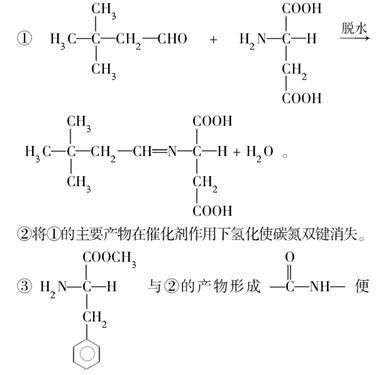
ÓŠ»ś»ÆŗĻĪļÓėČĖĄąÉś»īĆܲ»æÉ·Ö£¬Éś»īÖŠµÄŅ»Š©ĪŹĢā³£Éę¼°»ÆѧÖŖŹ¶”£
(1)ÓŠĻĀĮŠ¼øÖÖŹ³Ę·£ŗ
¢Ł»ØÉśÓĶÖŠĖłŗ¬ČĖĢåĖłŠčµÄÖ÷ŅŖÓŖŃųĪļÖŹĪŖ________(Ģī”°ĢĒĄą”±”°ÓĶÖ¬”±»ņ”°µ°°×ÖŹ”±)”£
¢Ś³Ō·¹Ź±£¬¾×½ĄĆ×·¹Ņ»»į¶łŗóøŠ¾õÓŠĢšĪ¶£¬ŹĒŅņĪŖµķ·Ū·¢ÉśĮĖ________·“Ó¦(Ń”ĢīĻĀĮŠŃ”ĻīŗÅ)”£
A£®·Ö½ā””””””B£®Ė®½ā””””””C£®ĮŃ½ā
(2)ŌŚČÕ³£Éś»īÖŠ£¬ĻĀĮŠ×ö·Ø“ķĪóµÄŹĒ________”£
A£®ÓĆČ¼Éշؼų±šĆ«ÖÆĘ·ŗĶĆŽÖÆĘ·
B£®ÓĆ“æ¼īĻ“µÓ¹ųøĒÉĻµÄÓĶ×Õ
C£®ÓĆĪÅĘųĪ¶µÄ·½·Ø¼ų±š°×¾ĘŗĶĆד×
D£®ÓƵķ·ŪČÜŅŗ¼ų±š¼ÓµāŹ³ŃĪŗĶ²»ŗ¬µāµÄŹ³ŃĪ
 ÅąÓÅæŚĖćĢāæØĻµĮŠ“š°ø
ÅąÓÅæŚĖćĢāæØĻµĮŠ“š°ø æŖŠÄæŚĖćĢāæØĻµĮŠ“š°ø
æŖŠÄæŚĖćĢāæØĻµĮŠ“š°ø æŚĖćĢāæØŗÓ±±ÉŁÄź¶łĶƳö°ęÉēĻµĮŠ“š°ø
æŚĖćĢāæØŗÓ±±ÉŁÄź¶łĶƳö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(14·Ö)õ„ĄąĪļÖŹA(C11H8O4)ŌŚĒāŃõ»ÆÄĘČÜŅŗÖŠ¼ÓČČ·“Ó¦ŗóŌŁĖį»ÆæɵƵ½»ÆŗĻĪļBŗĶC”£
»Ų“šĻĀĮŠĪŹĢā£ŗ
¢ŁBµÄ·Ö×ÓŹ½ĪŖC2H4O2£¬·Ö×ÓÖŠÖ»ÓŠŅ»øö¹ŁÄÜĶÅ”£ŌņBµÄ½į¹¹¼ņŹ½ŹĒ________£¬BÓėŅŅ“¼ŌŚÅØĮņĖį“ß»ÆĻĀ¼ÓČČ·“Ӧɜ³ÉD£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ________________________£¬øĆ·“Ó¦µÄĄąŠĶŹĒ________£»Š“³öĮ½ÖÖ¾ßÓŠČ©»ł£Ø”ŖCHO£©µÄBµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½________________________________”£
¢ŚCŹĒ·¼Ļć×å»ÆŗĻĪļ£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖ180”£ĘäĢ¼µÄÖŹĮæ·ÖŹżĪŖ60.0%£¬ĒāµÄÖŹĮæ·ÖŹżĪŖ4.44%£¬ĘäÓąĪŖŃõ£¬ŌņCµÄ·Ö×ÓŹ½ŹĒ________”£
¢ŪŅŃÖŖCµÄ±½»·ÉĻÓŠČżøöČ”“ś»ł”£ĘäÖŠŅ»øöČ”“ś»łĪŽÖ§Į“£¬ĒŅŗ¬ÓŠÄÜŹ¹äåµÄĖÄĀČ»ÆĢ¼ČÜŅŗĶŹÉ«µÄ¹ŁÄÜĶż°ÄÜÓėĢ¼ĖįĒāÄĘČÜŅŗ·“Ó¦·Å³öĘųĢåµÄ¹ŁÄÜĶÅ£¬ŌņøĆČ”“ś»łÉĻµÄ¹ŁÄÜĶÅĆū³ĘŹĒ________£¬ĮķĶāĮ½øöČ”“ś»łĻąĶ¬£¬·Ö±šĪ»ÓŚøĆČ”“ś»łµÄĮŚĪ»ŗĶ¶ŌĪ»£¬ŌņCµÄ½į¹¹¼ņŹ½ŹĒ________________”£Čē¹ūÖ»±ä»ÆĪļÖŹC±½»·ÉĻČżøöČ”“ś»łµÄĪ»ÖĆ£¬ŌņæÉŅŌµĆµ½________ÖÖCµÄĶ¬·ÖŅģ¹¹Ģ唣
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
°“ŅŖĒóĶź³ÉĻĀĮŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£¬²¢×¢Ć÷Ļą¹Ų·“Ó¦µÄ·“Ó¦ĄąŠĶ”£
(1)ĘĻĢŃĢĒÓėŅŅĖį×÷ÓĆÉś³ÉĘĻĢŃĢĒĪåŅŅĖįõ„
·½³ĢŹ½__________________________________________________________”£
·“Ó¦ĄąŠĶ________________________________________________________”£
(2)ĘĻĢŃĢĒÓėH2×÷ÓĆÉś³É¼ŗĮł“¼
·½³ĢŹ½___________________________________________________________”£
·“Ó¦ĄąŠĶ________________________________________________________”£
(3)ĘĻĢŃĢĒÓėŠĀÖĘCu(OH)2Šü×ĒŅŗ×÷ÓĆÉś³ÉŗģÉ«³Įµķ
·½³ĢŹ½__________________________________________________________”£
·“Ó¦ĄąŠĶ_________________________________________________________”£
(4)ÕįĢĒĖįŠŌĢõ¼žĻĀĖ®½ā
·½³ĢŹ½__________________________________________________________”£
·“Ó¦ĄąŠĶ________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
æĘѧ¼Ņ·¢ĻÖijŅ©ĪļMÄÜÖĪĮĘŠÄŃŖ¹Ü¼²²”ŹĒŅņĪŖĖüŌŚČĖĢåÄŚÄÜŹĶ·Å³öŅ»ÖÖ”°ŠÅŹ¹·Ö×Ó”±D£¬²¢²ūĆ÷ĮĖDŌŚČĖĢåÄŚµÄ×÷ÓĆŌĄķ£¬ĪŖ“ĖĖūĆĒČŁ»ńĮĖŵ±“¶ū»ÆѧŗĶŅ½Ń§½±”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)ŅŃÖŖMµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ227£¬ÓÉC”¢H”¢O”¢NĖÄÖÖŌŖĖŲ×é³É£¬C”¢H”¢NµÄÖŹĮæ·ÖŹżŅĄ“ĪĪŖ15.86%”¢2.20%ŗĶ18.50%”£ŌņMµÄ·Ö×ÓŹ½ŹĒ____________”£DŹĒĖ«Ō×Ó·Ö×Ó£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖ30£¬ŌņDµÄ·Ö×ÓŹ½ĪŖ____________________”£
(2)ÓĶÖ¬A¾ĻĀĮŠĶ¾¾¶æɵƵ½M”£
¶Ō¢ŚµÄĢįŹ¾£ŗ
C2H5OH£«HO”Ŗ NO2 C2H5O”Ŗ NO2£«H2O
C2H5O”Ŗ NO2£«H2O
ĻõĖį ĻõĖįŅŅõ„
·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½ŹĒ_________________________________________”£
·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ŹĒ_______________________________________”£
(3)CŹĒBŗĶŅŅĖįŌŚŅ»¶ØĢõ¼žĻĀ·“Ӧɜ³ÉµÄ»ÆŗĻĪļ£¬Ļą¶Ō·Ö×ÓÖŹĮæĪŖ134£¬Š“³öCĖłÓŠæÉÄܵĽį¹¹¼ņŹ½__________________________________________”£
(4)Čō½«0.1 mol BÓė×ćĮæµÄ½šŹōÄĘ·“Ó¦£¬ŌņŠčĻūŗÄ________ g½šŹōÄĘ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijӊ»śĪļA»ÆѧŹ½ĪŖCxHyOz,15 g AĶźČ«Č¼ÉÕæÉÉś³É22 g CO2ŗĶ9 g H2O”£ŹŌĒó£ŗ
£Ø1£©øĆÓŠ»śĪļµÄ×ī¼ņŹ½________”£
£Ø2£©ČōAµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ60ĒŅŗĶNa2CO3»ģŗĻÓŠĘųĢå·Å³ö£¬AŗĶ“¼ÄÜ·¢Éśõ„»Æ·“Ó¦£¬ŌņAµÄ½į¹¹¼ņŹ½ĪŖ________”£
£Ø3£©ČōAµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ60ĒŅŹĒŅ×»Ó·¢ÓŠĖ®¹ūĻćĪ¶µÄŅŗĢ壬ÄÜ·¢ÉśĖ®½ā·“Ó¦£¬ŌņĘä½į¹¹¼ņŹ½ĪŖ________________”£
£Ø4£©ČōA·Ö×Ó½į¹¹ÖŠŗ¬ÓŠ6øöĢ¼Ō×Ó£¬¾ßÓŠ¶ąŌŖ“¼ŗĶČ©µÄŠŌÖŹ£¬ŌņĘä½į¹¹¼ņŹ½ĪŖ_____________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø¹²12·Ö£©ĻÖÓŠĻĀĮŠÖÖĪļÖŹ£ŗ¢Ł CH3OH ¢Ś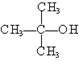
¢Ū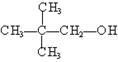 ¢Ü
¢Ü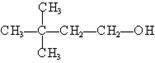 ¢Ż
¢Ż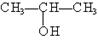
£Ø1£©ÄÜ·¢ÉśĻūČ„·“Ӧɜ³ÉĻ©ĢžµÄŹĒ £ØĒėĢīŠņŗÅ£¬ĻĀĶ¬£©£¬ĘäĻūČ„²śĪļŅĄ“ĪŹĒ £»ÓÉ“ĖæÉŅŌĶĘ³ö±½¼×“¼£Ø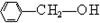 £© £ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©·¢ÉśĻūČ„·“Ó¦”£
£© £ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©·¢ÉśĻūČ„·“Ó¦”£
£Ø2£©ÄÜŌŚĶµÄ“ß»Æ×÷ÓĆĻĀ£¬±»æÕĘųÖŠŃõĘųŃõ»Æ³ÉČ©µÄŠņŗÅ·Ö±šŹĒ £¬ĒėŠ“³öÄܱ»Ńõ»Æ³ÉĶŖµÄ·“Ó¦·½³ĢŹ½£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø5·Ö£©”°¾ĘŹĒ³ĀµÄĻć”±£¬¾ĶŹĒŅņĪŖ¾ĘŌŚ“¢“ę¹ż³ĢÖŠÉś³ÉĮĖÓŠĻćĪ¶µÄŅŅĖįŅŅõ„£¬ŌŚŹµŃéŹŅĪŅĆĒŅ²æÉŅŌÓĆĻĀĶ¼ĖłŹ¾µÄ×°ÖĆÖĘČ”ŅŅĖįŅŅõ„”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öÖĘČ”ŅŅĖįŅŅõ„µÄ»Æѧ·“Ó¦·½³ĢŹ½£ŗ
______________________________________________________________
£Ø2£©±„ŗĶĢ¼ĖįÄĘČÜŅŗµÄÖ÷ŅŖ×÷ÓĆŹĒ_____________________________________________”£
£Ø3£©ČōŅŖ°ŃÖʵƵÄŅŅĖįŅŅõ„·ÖĄė³öĄ“£¬Ó¦²ÉÓƵďµŃé²Ł×÷ŹĒ_____________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ӊ׏ĮĻĻŌŹ¾£¬¼øÖÖĢšĪ¶¼ĮµÄĻą¶ŌĢš¶ČČēĻĀ£ŗ
| ĢšĪ¶¼ĮĆū³Ę | Ļą¶ŌĢš¶Č |
| ÕįĢĒ | 1 |
| ĢĒ¾« | 500”«700 |
| °¢Ė¹°ĶĢš | 180”«200 |
| ŦĢš | 7000”«13000 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŅŃÖŖÓŠ»śĪļAŗĶB¶¼Ö»ŗ¬C”¢H”¢OČżÖÖŌŖĖŲ”£
(1)AµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ62£¬A¾“ß»ÆŃõ»ÆÉś³ÉD£¬D¾“ß»ÆŃõ»ÆÉś³ÉE£¬AÓėEŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦æÉÉś³ÉŅ»ÖÖ»·×“»ÆŗĻĪļF”£
ŌņAµÄ·Ö×ÓŹ½ĪŖ________£¬½į¹¹¼ņŹ½ĪŖ________£»AÓėE·“Ӧɜ³ÉFµÄ·“Ó¦ĄąŠĶĪŖ________”£
(2)¶ŌÓŠ»śĪļBµÄ×é³É”¢½į¹¹½ųŠŠ·ÖĪö²ā¶Ø£¬µĆŹµŃé½į¹ūČēĻĀ£ŗ
¢ŁĶźČ«Č¼ÉÕ166 mgÓŠ»śĪļB£¬µĆµ½352 mg CO2ŗĶ54 mg H2O£»
¢Ś²ā³öBµÄŗĖ“Ź²ÕńĒāĘ×ÓŠ2øö·å£¬ŗģĶā¹āĘ×ĻŌŹ¾½į¹¹ÖŠŗ¬±½»·ŗĶōČ»ł£»
¢ŪBµÄĻą¶Ō·Ö×ÓÖŹĮæŌŚ100”«200Ö®¼ä”£
ŌņBµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ________£¬½į¹¹¼ņŹ½ĪŖ________”£
(3)AÓėBŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦æÉÉś³ÉŅ»ÖÖ³£¼ūŗĻ³ÉĻĖĪ¬£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com