
����пΪ��ɫ��ĩ��������ʪ�Ѣ��Ƥ���������ơ�������ҵ������п[����Fe(��)��Mn(��)��Ni(��)������]���������£�
��ҵZnO ����Һ
����Һ
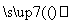
��
��Һ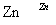
 ��Һ
��Һ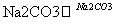
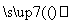 �˱�
�˱� ZnO
ZnO
��ʾ���ڱ�ʵ�������£�Ni(��)���ܱ�������������صĻ�ԭ������MnO2��
�ش��������⣺
(1)��Ӧ���г���������������__________��������Ӧ�����ӷ���ʽΪ__________���ڼӸ��������Һǰ����pH�ϵͣ��Գ��ӵ�Ӱ����________________��
(2)��Ӧ�۵ķ�Ӧ����Ϊ____________�����˵õ��������У����˹�����п���______________��
(3)��Ӧ���γɵij���Ҫ��ˮϴ����������Ƿ�ϴ�Ӹɾ��ķ�����________________��
(4)��Ӧ���в���ijɷֿ�����ZnCO3·xZn(OH)2��ȡ�������˱�11.2 g�����պ�ɵõ���Ʒ8.1 g����x����__________��
�𰸡�(1)Fe2����Mn2����MnO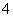 ��3Fe2����7H2O===3Fe(OH)3����MnO2����5H����2MnO
��3Fe2����7H2O===3Fe(OH)3����MnO2����5H����2MnO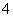 ��3Mn2����2H2O===5MnO2����4H���������Ӻ������Ӳ������ɳ������Ӷ�����ȥ����������
��3Mn2����2H2O===5MnO2����4H���������Ӻ������Ӳ������ɳ������Ӷ�����ȥ����������
(2)�û���Ӧ����
(3)ȡ����ˮϴҺ���Թ��У�����1��2��ϡ���ᣬ�ٵ������ᱵ��Һ�����ް�ɫ�������ɣ���˵�������Ѿ�ϴ�Ӹɾ�
(4)1
�������ӷ�����������ͼ���֣���ȷÿ�������ķ�Ӧ���Ӷ����������⡣
(1)�ڷ�Ӧ���У�ͨ��������Һ��pH����������ܽ���Һ�е�Fe2������ΪFe3������Mn2������ΪMnO2����ȥ������Һ��pH���ͣ�Fe2����Mn2�����������ɳ�������ȥ��
(2)��һ�ι��˺����Һ�к��е���������Zn2����Ni2����H���ȣ�����п��ɽ�Ni�û��������������л����н�������
(3)��Ӧ�����ɵij���ΪZnCO3��ͬʱ����Na2SO4��������δϴ�Ӹɾ���ϴ��Һ��Ӧ����SO ��Na������ֻҪ��ϴ��Һ���Ƿ���SO
��Na������ֻҪ��ϴ��Һ���Ƿ���SO ���м��鼴�ɡ�
���м��鼴�ɡ�
(4)���չ�����ZnCO3��Zn(OH)2�������ֽⷴӦ����ZnO�����ݹ�ϵʽZnCO3·xZn(OH)2��(x��1)ZnO���ɵ�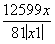 ��
��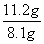 ����x��1��
����x��1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ȼ��ⱥ��ʳ��ˮ��ȡNaOH��Һ�Ĺ�����������ͼ��ʾ�����������գ�
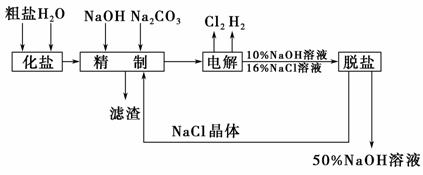
(1)�ڵ������У����Դ���������ĵ缫�ϵĵ缫��ӦʽΪ________________________________________________________________________��
���������Դ���������ĵ缫�ϵ�������ʵ������ͨ��ѡ�õĻ�ѧ�Լ���________________________________________________________________________��
(2)��ҵʳ���к�Ca2����Mg2�������ʣ����ƹ��̷�����Ӧ�����ӷ���ʽΪ________________________________________________________________________��
(3)���������SO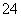 �����ϸߣ��������ӱ��Լ���ȥ���ñ��Լ�������________(ѡ��a��b��c)��
�����ϸߣ��������ӱ��Լ���ȥ���ñ��Լ�������________(ѡ��a��b��c)��
a��Ba(OH)2��b��Ba(NO3)2��c��BaCl2
(4)Ϊ����Ч�س�ȥCa2����Mg2����SO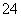 �������Լ��ĺ���˳��Ϊ________(ѡ��a��b��c)��
�������Լ��ĺ���˳��Ϊ________(ѡ��a��b��c)��
a���ȼ�NaOH�����Na2CO3���ټӱ��Լ�
b���ȼ�NaOH����ӱ��Լ����ټ�Na2CO3
c���ȼӱ��Լ������NaOH���ټ�Na2CO3
(5)���ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ��________����ȴ��________(��д��������)��ȥNaCl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
P2O5�Ƿ������Ը�������������岻����Ũ����������P2O5�������________��
a��NH3 b��HI c��SO2 d��CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)____HCl(Ũ)��____MnO2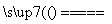 ____Cl2����____MnCl2��____H2O
____Cl2����____MnCl2��____H2O
(2)____Cu��____HNO3(ϡ)===____Cu(NO3)2��____NO����____H2O
(3)____KI��____KIO3��____H2SO4===____I2��____K2SO4��____H2O
(4)____MnO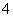 ��____H����____Cl��===____Mn2����____Cl2����____H2O
��____H����____Cl��===____Mn2����____Cl2����____H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��P��CuSO4��H2O����Cu3P��H3PO4��H2SO4(δ��ƽ)�ķ�Ӧ�У�7.5 mol CuSO4������P�����ʵ���Ϊ________mol������1 mol Cu3Pʱ���μӷ�Ӧ��P�����ʵ���Ϊ________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������л�ԭ�ԣ����Ժ�������������Ӧ�������������£�H2S��KMnO4��Ӧ����S��MnSO4��K2SO4��H2O��д���÷�Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������A��B��C��D��E�����ǵ������ӿ�����Na����NH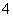 ��Cu2����Ba2����Al3����Ag����Fe3���������ӿ�����Cl����NO
��Cu2����Ba2����Al3����Ag����Fe3���������ӿ�����Cl����NO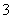 ��SO
��SO ��CO
��CO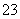 ����֪��
����֪��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ�ʻ�ɫ��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԡ�
�������������ε���Һ�зֱ����Ba(NO3)2��Һ��ֻ��A��C����Һ������������
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��
�ް�A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����
��ش��������⣺
(1)�������У�һ��û�е���������____________��������������ͬ�������εĻ�ѧʽ��__________________��
(2)D�Ļ�ѧʽΪ__________________��D��Һ�Լ��Ե�ԭ����________________________(�����ӷ���ʽ��ʾ)��
(3)A��C����Һ��Ӧ�����ӷ���ʽ��_______________________________��
E�Ͱ�ˮ��Ӧ�����ӷ���ʽ��____________________________________________��
(4)��Ҫ����B�������������ӣ���ȷ��ʵ�鷽����___________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com