
�����ʵ�����Ϊ0.1 mol��CuCl2��H2SO4����ˮ�Ƴ�100 mL�Ļ����Һ����ʯī���缫��⣬���ռ����缫�����������壬һ��ʱ����������ռ�������������ͬ�����������ͬ��������������ȷ���� (����)
A����·�й�ת��0.6NA������
B�������õ���������O2�����ʵ���Ϊ0.2 mol
C��������������3.2 g
D������ʣ����Һ�������Ũ��Ϊ1 mol��L��1

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и����ʵ�������ȷ����
A��1��2���������� B��2��3��3����������
C��2��3��3���������� D��2��3������-4-�һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������Ե缫����500 mL AgNO3��Һ�У�ͨ���⡣�����Һ��pH��6.0��Ϊ3.0ʱ(�������������û��H2�ų����ҵ��Һ�ڵ��ǰ������仯���Ժ��Բ���)���缫�����������������Ϊ (����)
A��27 mg B��54 mg
C��106 mg D��216 mg
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ը����Ϊԭ�ϣ��绯ѧ���Ʊ��ظ���ص�ʵ��װ��ʾ��ͼ���£�
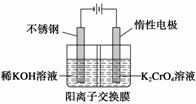
����˵������ȷ���� (����)
A���������ң������ĵ缫��ӦΪ2H2O��2e��===2OH����H2��
B���������ң�ͨ�����Һ���ɻ�ɫ��Ϊ��ɫ������Ϊ������H��Ũ������ʹƽ��2CrO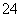 ��2H��Cr2O
��2H��Cr2O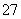 ��H2O�����ƶ�
��H2O�����ƶ�
C�����Ʊ��������ܷ�Ӧ�Ļ�ѧ����ʽΪ4K2CrO4��4H2O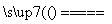 2K2Cr2O7��4KOH��2H2����O2��
2K2Cr2O7��4KOH��2H2����O2��
D���ⶨ����Һ��K��Cr�ĺ�������K��Cr�����ʵ���֮��(nK/nCr)Ϊd�����ʱ����ص�ת����Ϊ1��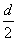
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1828�꣬�¹���ѧ��ά�գ�ʹ��һ������ֱ��ת��Ϊ�л������أ���һ�ɹ���Ϊ�л���ѧ����̱���ά��ʹ�õ������ǣ� ��
A��(NH4)2CO3 B��NH4NO3 C��NH4CNO D��CH3COONH4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʵķе㰴�ɸߵ��͵�˳��������ȷ���ǣ� ��
��CH3(CH2)2CH3 ��CH3(CH2)3CH3 ��(CH3)3C CH3 ��(CH3)2CHCH2CH3
A���ڢܢ٢� B���ܢڢ٢�
C���ܢۢڢ� D���ڢܢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ����ᡢ����������ء��������������ʵ�Ľ�����ȷ����
| ѡ�� | �������ʵ | ���� |
| A | ���ȵ��ռ���Һϴȥ���� | Na2CO3��ֱ�Ӻ����۷�Ӧ |
| B | Ư���ڿ����о��ñ��� | Ư���е�CaCl2 ������е�CO2��Ӧ����CaCO3 |
| C | ʩ��ʱ����ľ��(��Ч�ɷ�ΪK2CO3)������NH4Cl���ʹ�� | K2CO3��NH4Cl��Ӧ���ɰ����ή�ͷ�Ч |
| D | FeCl3��Һ������ͭ��ӡˢ��·������ | FeCl3�ܴӺ���Cu2������Һ���û���ͭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ĵ��ӽṹ�У���һ��������С��ԭ���������(����)
A.ns2np3 B.ns2np4 C.ns2np5 D.ns2np6
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com