
��δ�ʯ���л�ø��������ȼ��һֱ�ǻ�ѧ��̽���Ŀ��⣬��ʯ�ͷ���õ������ͽ�����
�����Ի�ø��������ȼ�͡�
����һ��ʯ���Ǻ���20��30��̼ԭ�ӵ������Ļ��������³ʹ�̬��
���϶���ʯ�ʹ� �ѻ���ͨ��ʹ��Al2O3��������
�ѻ���ͨ��ʹ��Al2O3��������
ij�о��� ѧϰС����ʵ������ģ��ʯ�͵Ĵ��ѻ���װ����ͼ��ʵ������пɹ۲쵽��ƿ���й���ʯ�����ۻ����Թܢ���������Һ�����ᣬ�Թܢ������Ը��������Һ��ɫ��ʵ������Թܢ���Һ����ζ���������͵���ζ��
ѧϰС����ʵ������ģ��ʯ�͵Ĵ��ѻ���װ����ͼ��ʵ������пɹ۲쵽��ƿ���й���ʯ�����ۻ����Թܢ���������Һ�����ᣬ�Թܢ������Ը��������Һ��ɫ��ʵ������Թܢ���Һ����ζ���������͵���ζ��
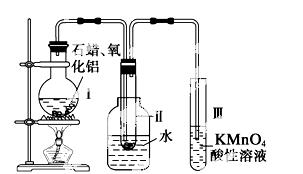

��1����װ���������ӵ�˳��Ӧ��ѭ��ԭ��Ϊ__________________________________��Ϊ��֤ʵ��ɹ���ʵ��ǰ������еIJ�����__________________________________��װ���нϳ����ܵ�������______________��
��2���Թܢ����� ����Һ������˵����_____________��
����Һ������˵����_____________��
��3���Թܢ�����Һ��ɫ˵����___________________��
��4���ܷ����Թܢ��е�Һ����ȡ��ˮ�е��壬������_________________________________________��
��5��д����ʮ���ѻ��õ������ϩ�Ļ�ѧ����ʽ___________________________��
��6�� ʯ���ѻ�����Ҫ������__________________��
ʯ���ѻ�����Ҫ������__________________��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ǿ����������£���28.6 g  CaClO2��Ư������Һ��ͨ��1.12 L CO2��״�������ɲ�����״���µ��������������
CaClO2��Ư������Һ��ͨ��1.12 L CO2��״�������ɲ�����״���µ��������������
A��1.12 L B��2.24 L C��3.36 L D��4.48 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����¹����з���ˮ�ⷴӦ����(����)
A����֯���Ũ������
B��Ƥ����մ��Ũ�������
C��������֮�䷴Ӧ�����ɶ���
D�����������ʵ����������ɰ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
�ٱ�״���£�0.2 mol�κ����ʵ������Ϊ4.48 L������1 mol��������Ϊ22.4 L������һ�����ڱ�״���¡��۱�״���£�1 L HCl��1 L H2O�����ʵ�����ͬ���ܱ�״���£�1 g H2��14 g N2�������ͬ����28 g CO�����Ϊ22.4 L�����������ʵ����ʵ�����ͬ���������ڱ�״���µ����Ҳ��ͬ������ͬ��ͬ���ʱ���������ʵ����ʵ���Խ����ѹǿԽ��ͬ��ͬѹ�£�������ܶ����������Է�������������
A���٢ڢۢ� B���ڢۢޢߢ�
C���ݢޢߢ� D���ܢߢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ����Ҫ��CuSO4·5H2O��������500 mL 0.1 mol·L��1 CuSO4��Һ���ش��������⣺
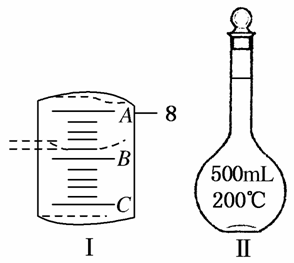
(1)Ӧ����������ƽ��ȡCuSO4·5H2O________g��
(2)��ͼ���ʾ10 mL��Ͳ��Һ���λ�ã�A��B��B��C�̶ȼ����1 mL������̶�AΪ8����Ͳ��Һ��������________mL��
(3)��ʵ������ͼ����ʾ�������������������������Һ��Ũ���к�Ӱ�죿(�ƫ�ߡ���ƫ�͡�����Ӱ�족)
A������ǰ����ƿ�ײ���ˮ��__________________________��
B������ʱ��ˮ�����̶���____________________________��
C�����ն���ʱ���ӹ۲�Һ��__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ѧ������������á���������������ֱ��������һ��ּ����(����)
A���������磬����Դ����ࡡ�� B���ϳɹ��ˣ���ͨѶ�����
C���ϳ�ҩ������������ D������Ϳ�ϣ��û������˾�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ⱦ����Խ��Խ�ܵ����ǵĹ�ע,��ɻ�����Ⱦ����Ҫԭ����������������й����ŷ��й���������ġ���������������Ӧ�����ʲ���ص���(����)
A������ЧӦ——������̼
B���⻯ѧ��Ⱦ——��������
C������——������̼
D����ɫ��Ⱦ——һ����������Ʒ��ʹ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
20-I��6�֣�����������ȷ����
A���ϳɰ��ġ��������λ��������
B����Ƶ����Է�Һ�ü��кͺ�Ϳ����ŷ�
C����������Ĺ����У���Ϊ�������ϵ�����ú��������
D��ʹ��ú̿ת���Ĺܵ�ú����ֱ��ȼú�ɼ��ٻ�����Ⱦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1966����������������˾�״η�����Na��S��ص��йر�������ṹ��ͼ��ʾ����ط�ӦΪ2Na��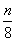 S8
S8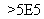 Na2Sn������˵������ȷ����
Na2Sn������˵������ȷ����
A�����Ƶ缫����ص�����
B���ŵ�ʱNa���������ƶ�
C�����ʱ���Ƶ缫���Դ�ĸ�������
D�����ʱ������ӦʽΪ8S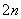 —16e��=nS8
—16e��=nS8
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com