
����Ŀ������������Ԫ��R��X��Y��Z��ԭ��������������R�������̬�⻯����ӵĿռ�ṹΪ�������壬X��+1�������ӵĵ��Ӳ�ṹ����ԭ����ͬ��Ԫ��X��Z�γɻ�����G��G��X��ZԪ�ص�����֮��Ϊ23�U16�������ֺ�YԪ�صĿ����Ի�����E��F����10mL1.0mol��L��1E��Һ�еμ�1.0mol��L��1F��Һ���������������ʵ���(n)��F��Һ���(V)�Ĺ�ϵ��ͼ��ʾ������˵��һ����ȷ����

A. ԭ�Ӱ뾶��X>Y>R
B. ����������Ӧˮ��������ԣ�Z>Y>R
C. X��Y�ĵ�����ɵĻ���ﲻ����ȫ����ˮ
D. ��ҵ�ϣ�ͨ����������Ȼ����Ʊ�Y�ĵ���
���𰸡�A
��������
�����������֪��X��+1�������ӵĵ��Ӳ�ṹ����ԭ����ͬ���Ƶ�XΪ�ƣ�R�������̬�⻯����ӵĿռ�ṹΪ�������壬RΪ̼��Ԫ�أ����ԭ��������ϵ��RΪ̼��Ԫ��X��Z�γɻ�����G��G��X��ZԪ�ص�����֮��Ϊ23�U16��ZӦΪ��ͨ��ͼ���֪E:F:����=1:3:4�����Ƶ�YΪ����
A.������ͬ���ڣ��Ƶ���ԭ�Ӱ뾶��������̼λ���ƺ�������һ���ڣ����뾶С����A��ȷ��
B.̼������������Ӧˮ����Ϊ̼�ᣬΪ����������������Ӧˮ����Ϊ���ᣬΪǿ���������������Ӧˮ����Ϊ����������Ϊ�����������������������B����
C.�Ƶ�����������Ͷ��ˮ�У����ƹ�������ˮ��Ӧ�����ɴ����������ƣ����Խ���ȫ���ܽ⣬��C����
D.��ҵ�ϲ��õ���������������Ʊ������ʣ��������Ȼ���Ϊ���ۻ�������ڲ����磬���ܱ���⣬��D����
�ۺ����Ϸ����������ΪA��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ϳ������ה������ġ��������ۡ�����¼��һ�ֿ�����ҩ��ʯ��Ǧ���ֳ���Ȼͭ�����л�Ѫ����ֹʹ��Ч���������ִ������������һ��ʯ��Ǧ��Ʒ�������京ͭ���٣�������Ԫ�ص���������Ϊ44.8������Ԫ�ص���������Ϊ51.2�����ش��������⣺
(1)ʯ��Ǧ����Ҫ�ɷ���һ�ֻ�����(��������Ϊ96��)����ѧʽΪ_________��ʯ��Ǧ��ĩ��������ȫ���������ữ�ĸ��������Һ�У��γɶ��������εĻ��Һ��д����Ҫ��Ӧ�����ӷ���ʽ��______________________��
(2)����ʯ��Ǧ����̿�������ڸ����·�Ӧ��������ǣ�����ƷΪFe3O4��CO��Fe3O4��_____ɫ���壬������������ӡ����ī�ۣ�CO�����ںϳ��ڷ�֯��ҵ�й㷺ʹ�õı��շ�(Na2S2O4)�����������£�

�ϳ���I�еõ���HCOONa��Һ������������ԼΪ5�������з����HCOONa��2H2O����Ҫ�����ǡ�______�����ˡ�ϴ�ӡ�����ϳ���II�з�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ____________�����շ۱�¶�ڿ�����������������ˮ���������ʣ��������������ʵ���һ���������һ����ˮ����ʱ�������γ�____________(�ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ú����Ҫ��Դ��ȼú���ͷ�SO2��CO���ж����塣�����ж�������о���ʵ����δֹͣ�����������գ�
I.��CaSO4����CO��CaSO4��CO�ɷ�������������Ӧ��
��Ӧ�٣�CaSO4��s��+4CO��g��![]() CaS��s��+4CO2��g��+175.6kJ
CaS��s��+4CO2��g��+175.6kJ
��Ӧ�ڣ�CaSO4��s��+4CO��g��![]() CaO��s��+SO2��g��+CO2��g����218.4kJ
CaO��s��+SO2��g��+CO2��g����218.4kJ
(1)д����Ӧ�ٵ�ƽ�ⳣ������ʽ��_____________��һ�������µ��ܱ������У�����Ӧ����ƽ�����ʹ��Ӧ��K�ļ�С����Ҫ�ı�ķ�Ӧ������______________����Ӧ�ڵ�K___________��ѡ���ţ�����Ӧ�ٵ�v(CO2)��__________��ѡ���ţ���
a. ���� b. ��С c. ���� d. ���ж�
��2����ȫ��ͼ�з�Ӧ�ڵ������仯ʾ��ͼ_______________������ע�������������Ĵ���λ�ü���Ӧ��ЧӦ��ֵ��

��3����ͼ�Dz�ͬ�¶��£�CO��ʼ����ٷ�����ƽ��ʱ���������CaS�����ٷ����Ĺ�ϵ���ߡ�д�����ֽ���SO2�������Ĵ�ʩ___________________________________��
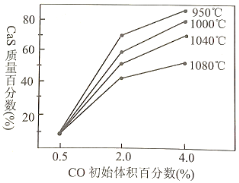
II. Fe2(SO4)3��Һ�ɳ�ȥú���Ի�����FeS2����ʽ���ڵ���Ԫ�أ���Ӧ���£�8H2O + FeS2+ 7Fe2(SO4)3��15FeSO4+ 8H2SO4
(4)�������ת�Ƶ���Ŀ�ͷ���________����ԭ������_______��
(5)����������Ӧ��Fe2(SO4)3�Ƿ��������ʵ�鷽����___________________________��
(6)�÷������ŵ�֮һ��Fe2(SO4)3����������Ӧ�����Һ��ͨ��___________�����ܴﵽʹFe2(SO4)3������Ŀ�ģ������Ҽ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������һ�������£������Ϊ3 L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO(g) �� 2 H2(g) ![]() CH3OH(g)
CH3OH(g)
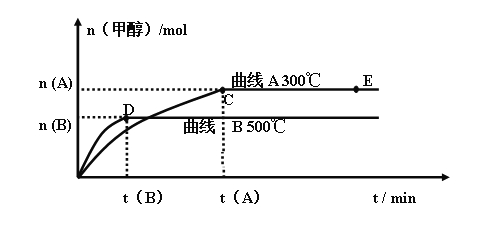
��ͬ�¶�ʱ�״������ʵ�����ʱ��仯��������ͼ��ʾ����������������и��⣺
(1)��Ӧ�ﵽƽ��ʱ�������¶ȣ���ѧƽ�ⳣ��Kֵ____________������������������С����������������
(2)�������������������£���E�����ϵ���ѹ����ԭ���� �������йظ���ϵ��˵����ȷ����___________
a ������Ũ�ȼ�С b ����Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c �״������ʵ������� d ����ƽ��ʱn(H2)/n(CH3OH)���� e ƽ�ⳣ��K����
����1 L���ܱ������У����з�Ӧ����CO2(g)+H2(g) ![]() CO(g)+H2O(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
CO(g)+H2O(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
T������ | 700 | 800 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.7 | 2.6 |
(1) ��0.1 mol CO��0.1 mol H2O��ϼ��ȵ�800�棬һ��ʱ���÷�Ӧ�ﵽƽ�⣬���CO2�����ʵ���Ϊ0.053 mol������������������830�棬ƽ��ʱCO2�����ʵ���_____ (������������������������С����)0.053 mol��
(2)800��ʱ������CO��H2O��CO2��H2�������ʵ����ֱ�Ϊ��0.01��0.01��0.01��0.01mol����ӦCO2(g)+H2(g) ![]() CO(g)+H2O(g)��_________ (��������Ӧ�������淴Ӧ��)������С�
CO(g)+H2O(g)��_________ (��������Ӧ�������淴Ӧ��)������С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����һ�ڡ��������ۿ챨�������˿�ѧ�ҳɹ��Ʊ��˰����Ƽء��������Լ���������CaK(Fe1-xNix)4As4���ͻ�������ϡ�
�ش��������⣺
(1)��̬��ԭ�ӵĵ����Ų�ʽΪ[Ar]______________________����������������Ԫ�صĻ�̬ԭ���У�δ�ɶԵ�����������___________(��Ԫ�ط���)��
(2)AsCl3����������ӻ�������___________��AsO43���Ŀռ乹����___________��
(3)���������Ƿ�ӳԪ�����ʵIJ���֮һ����������ָ��̬��̬ԭ�ӵ�1�������γɸ�һ��������ʱ�ͷŵ�����(kJ��mol��1)��������������Ԫ��Ga��Ge��As��Se��Br�ĵ������ܴ�С�仯��ͼ��ʾ����ĵ������ܡ�ͻ�䡱����Ҫԭ����_________________________________��

(4)�������������γ������ӣ���[Fe(CN)6]3����[Fe(CN)6]4����Fe(CO)5�ȡ���λԭ���ṩ�µ��ӶԵ�������Ԫ�صĵ縺�Դ�С�йأ��縺��Խ����ԭ�Ӳ������ṩ�µ��Ӷԣ���Fe(CO)5���ṩ�µ��ӶԵ�ԭ����__ (��Ԫ�ط���)����CO��Ϊ�ȵ�����ķ�����___________��
(5)��֪��CaO��K2S���۵�ֱ�Ϊ2572�桢840�棬�����۵�������Ҫԭ����___________��
(6)������������ͼ1��ʾ����ԭ�Ӳ��������Ľṹ��ͼ2��ʾ��

��ͼ1�У�ԭ�����������A(0��0��0)��B(![]() ��0��
��0��![]() )����Cԭ�ӵ��������Ϊ___________��
)����Cԭ�ӵ��������Ϊ___________��
��ͼ2�У���֪�����Ӱ뾶Ϊapm(���������Ӱ뾶)��NA�ǰ����ӵ�������ֵ��ÿƽ����������������Ϊ___________g��(��ʾ����ͼ2���������и��������������С��Ԫ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D���ֻ�����ֱ���K+��Ba2+��SO��2����CO32��OH���е�������ɣ����Ǿ����������ʣ�
��A������ˮ�������B������ˮ�����������ᣬ���ų���ɫ�̼�����ζ������E����C��ˮ��Һ�ʼ��ԣ������ᷴӦ����A����D������ˮ������������ʱ�ų�����E��E��ʹ����ʯ��ˮ����ǡ�
(1)�ƶ�A��B��C��D�Ļ�ѧʽ��
A��________��B��________��C��________��D��________��
(2)д�����з�Ӧ�����ӷ���ʽ��
B�����ᷴӦ��_______________________________________________��
C�����ᷴӦ��_______________________________________________��
E(����)�����ʯ��ˮ��Ӧ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��������������ԭ�����͵��ǣ� ��
����ˮ��������ƽ��Br2��H2O![]() HBr��HBrO����������������Һ����Һ��ɫ��dz
HBr��HBrO����������������Һ����Һ��ɫ��dz
�ڹ�ҵ�ϳɰ���ӦN2(g) ��3H2(g)![]() 2NH3(g) ��H��0��Ϊ��߰��IJ��ʣ�ʵ�������в�ȡ���¡���ѹ�Ĵ�ʩ
2NH3(g) ��H��0��Ϊ��߰��IJ��ʣ�ʵ�������в�ȡ���¡���ѹ�Ĵ�ʩ
�۷�Ӧ2 NO2(g) ![]() N2O4(g)��ƽ�����С���������ʹ��ϵ��ɫ�ȱ�����dz
N2O4(g)��ƽ�����С���������ʹ��ϵ��ɫ�ȱ�����dz
�ܶ���2HI(g) ![]() H2(g) ��I2(g)����ƽ�����С���������ʹ��ϵ��ɫ����
H2(g) ��I2(g)����ƽ�����С���������ʹ��ϵ��ɫ����
A. �٢� B. �ڢ� C. �ۢ� D. �ڢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com