
��.ij�¶�ʱ����2 L�����У�ijһ��ѧ��Ӧ��A��B�����ʵ�����ʱ��仯�������� ͼ��ʾ����ͼ�����ݷ����ã�
ͼ��ʾ����ͼ�����ݷ����ã�

��1����4 minĩʱ��A��B�����ʵ���Ũ��c(A)________c(B)����0��4 min��A��B�����ʵ���Ũ�ȱ仯����c(A)________��c(B)(�����>����<������)��
��2���ӷ�Ӧ��ʼ��4 minʱ��A��ƽ����Ӧ����Ϊ________��
��3���÷�Ӧ�Ļ�ѧ����ʽΪ__________________��
��.�������ʵ�����A��B��Ϸ���2 L���ܱ������У�������Ӧ3A(g)��B(g)==X C(g)��2D(g)����5 min����D��Ũ��Ϊ0.5 mol��L��1��c(A)��c(B)��3��5��v(C)��0.1 mol��L��1��min��1��
��1��X��________��
��2��ǰ5 min��B�ķ�Ӧ����v(B)��________��5 minʱA��ת����Ϊ________��
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
������A��B�ֱ�Ϊ0.6mol��0.5mol����0.4L�ܱ������з�����Ӧ��3A+B mC+2D����5min��ﵽƽ�⣬��ʱCΪ0.2mol����֪�ڴ˷�Ӧʱ��D��ƽ����Ӧ���� Ϊ0.1mol��L-1��min-1�����½�����ȷ���ǣ� ��
mC+2D����5min��ﵽƽ�⣬��ʱCΪ0.2mol����֪�ڴ˷�Ӧʱ��D��ƽ����Ӧ���� Ϊ0.1mol��L-1��min-1�����½�����ȷ���ǣ� ��
A��mֵΪ3 B��B��ת����Ϊ20��
C��A��ƽ����Ӧ����Ϊ0.1mol��L-1��min-1 D��ƽ��ʱ��Ӧ����������ʵ���Ϊ1mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�����и߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���й��ڻ�ѧ���뻯�����������ȷ���ǣ� ��
�����ӻ�������һ�����н���Ԫ�� �ڹ��ۻ�������һ�������н���Ԫ�� �����ӻ�������һ���������Ӽ������ӻ�������һ�������й��ۼ� �����ӻ������п��ܺ��й��ۼ����ۻ������п��ܺ������Ӽ��߹��ۻ�������һ�����������Ӽ�
A���٢ݢޢ� B���ڢۢ� C���ۢݢ� D���ڢۢޢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�߶��ϵ�һ�ζο���ѧ���������棩 ���ͣ�ѡ����
��һ���������İ���������������Ƶ��ܱ���������У���������������䣬��������������Բ��ƣ���ʹ��ﵽ�ֽ�ƽ�⣺NH2COONH4(s)  2NH3(g)+CO2(g)��ʵ���ò�ͬ�¶��µ�ƽ�����������±���
2NH3(g)+CO2(g)��ʵ���ò�ͬ�¶��µ�ƽ�����������±���
�¶�/�� | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 |
ƽ����ѹǿ/kPa | 5.7 | 8.3 | 12.0 | 17.1 | 24.0 |
ƽ��������Ũ��/10-3mol/L | 2.4 | 3.4 | 4.8 | 6.8 | 9.4 |
�����й�������ȷ����
A���ÿ��淴Ӧ�ﵽƽ��ı�־֮һ�ǻ������ƽ����Է�����������
B����÷�Ӧ�ر䣨��S������0���ʱ䣨��H������0�������ڵ������Է�����
C�����ݱ������ݣ�����15.0��ʱ�ķֽ�ƽ�ⳣ��ԼΪ2.0��10��9(mol��L��1)3
D���ﵽƽ������ں�����ѹ�������������������粒����������С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꽭��ʡ�߶��ϵ�һ�ζο���ѧ���������棩 ���ͣ�ѡ����
���л�ѧ����ʽ�У���ȷ����
A�������ȼ���ȡ�H=��890.3kJ/mol�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��
CH4(g)+2O2(g)=CO2(g)+2H2O(g) ��H=��890.3kJ/mol
B��һ�������£���0.5molN2��1.5molH2�����ܱ������г�ַ�Ӧ����NH3����akJ�����Ȼ�ѧ����ʽΪ��N2(g)+3H2(g)  2NH3(g) ��H=��2akJ/mol
2NH3(g) ��H=��2akJ/mol
C����101kPaʱ��2gH2��ȫȼ������Һ̬ˮ���ų�285.8kJ����������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ��2H2(g)+O2(g)=2H2O(l) ��H=��571.6kJ/mol
D��HCl��NaOH��Ӧ���к��ȡ�H=��57.3kJ/mol����H2SO4��Ba(OH)2��Ӧ���к��ȡ�H=��114.6kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�걱���к��Ϸ�У�߶��ϵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
���ܱ������A��B��Ӧ����C���䷴Ӧ���ʷֱ���v(A)��v(B)��v(C)��ʾ����֪3v(B)��2v(A)��2v(C)��3v(B)����˷�Ӧ�� ��ʾΪ( )
��ʾΪ( )
A��2A��3B = 2C B��A��3B = 2C C��3A��2B = 3C D��A��B = C
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�걱���к��Ϸ�У�߶��ϵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
���ڻ�ѧ��Ӧ��������˵����ȷ����( )
A����ѧ��������Ҫ��������
B����ѧ��Ӧ�������仯�Ĵ�С�뷴Ӧ�������������
C���ڻ�ѧ��Ӧ�����з�Ӧ������������ǵ����������������
D�����ȷ�Ӧ�����Է����У����ȷ�Ӧ��Ҫ�ڼ��ȵ������²��ܽ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶��ϵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
ά����C(C6H8O6)��Ҫ�������߲˺�ˮ���У����ܴٽ�����������������ǿ����Լ����ĵֿ���������������ѧ�ҷ���ά����C�з������á����й���ά����C��˵���д�����ǣ�˫ѡ�������� ��
A������C������ ��
��
B��ά����C��6��̼Ԫ�ء�8����Ԫ�ء�6����Ԫ�����
C��ά����C��C��H��O����Ԫ�ص����� ��Ϊ9:1:12
��Ϊ9:1:12
D��������Ӧ����߲�ˮ�����м�ƫʳ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�걱���к��Ϸ�У��һ�ϵ�һ���¿���ѧ���������棩 ���ͣ�ʵ����
ijͬѧ��ij�ִ��ν����ᴿʵ�飬�������ͼ��
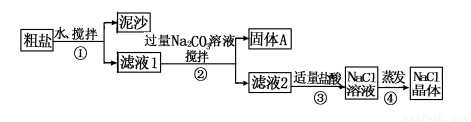
��ش�
(1)����ٺ͢ڵIJ���������________________��
(2)������жϼ������ᡰ�������ķ�����________________________��
����ܼ�������ʱҪ�ò��������Ͻ��裬����Ϊ�˷�ֹ______________�������������н϶����������ʱ��Ӧ________________��������ʹˮ�����ɡ�
(3)�������֤��
���� | ��֤�ķ��� | ���� | ���� |
�������A�к�CaCO3��MgCO3 | ȡ | �������� | |
�������A�к�BaCO3 | ȡ��������A���Թ��У��ȵ���________���ٵ���Na2SO4��Һ | �����ݷų����ް�ɫ���� | |
���������Ƶõ�NaCl�����л�����Na2SO4 | ȡ����NaCl���������Թ��е�����ˮ��______________ | �������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com