
��pH=4�����ᡢ���ᡢ������ƿ��Һ�������¾���>��<��=��ʾ��
��1����������Һ�����ʵ���Ũ������Ϊc1��c2��c3�������ϵ��
��2��ȡ��ͬ�����������ֱ��������ˮϡ�͵�pH=6����ˮ���������ΪV1��V2��V3�������ϵ�� ��
��3����ȫ�к���������ʵ���Ũ�Ⱦ���ͬ��Ba��OH��2��Һʱ��������������ʵ�������Ϊn1��n2��n3�������ϵ�� ��
��4��ȡͬ����������Һ�ֱ����������п�ۣ���Ӧ��ʼ�ų�H2����������Ϊ��1����2����3�������ϵ�� ����Ӧ�����зų�H2����������Ϊ��a����b����c�������ϵ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
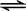 2NH3��g����H=-92kJ?mol-1
2NH3��g����H=-92kJ?mol-1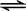 2NH3��g����H=-92kJ?mol-1
2NH3��g����H=-92kJ?mol-1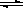 Ba2+��aq��+SO42-��aq��
Ba2+��aq��+SO42-��aq��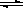 Ba2+��aq��+SO42-��aq��
Ba2+��aq��+SO42-��aq��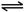 H++HCO3-
H++HCO3-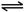 H++HCO3-
H++HCO3-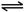 H++NH3?H2O
H++NH3?H2O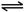 H++NH3?H2O
H++NH3?H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
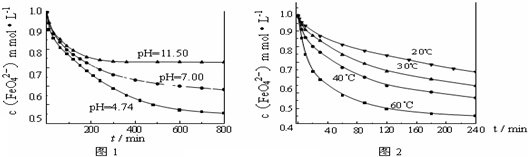

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
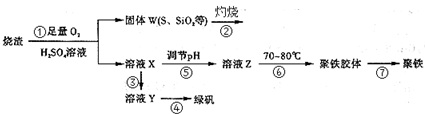
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?�����ģ��X��Y��Z��W��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮���ת����ϵ��ͼ1��ʾ��ˮ�����ֲ�������ȥ����
��2013?�����ģ��X��Y��Z��W��Ϊ��ѧ��ѧ�г����ĵ��ʻ������֮���ת����ϵ��ͼ1��ʾ��ˮ�����ֲ�������ȥ���� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
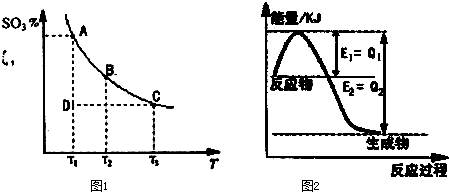
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com