
| |||||||||||||||

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | �����Լ� | ʵ������ |
| ��һ����Һ | �μ������ĵ���KI��Һ | ����ɫ |
| �ڶ�����Һ | �μ��������λ���BaCl2��Һ | �а�ɫ���� |
| ��������Һ | �μ���Һ�����ȣ����������������Һ�����V�������ɵij����������������ϵ��n������ͼ�� | 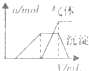 |
| ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
| c��NO2��/mol?L-1 | 1.00��10-3 | 4.50��10-4 | 2.50��10-4 | 1.50��10-4 | 1.00��10-4 | 1.00��10-4 |
| c��SO2��/mol?L-1 | 3.60��10-3 | 3.05��10-3 | 2.85��10-3 | 2.75��10-3 | 2.70��10-3 | 2.70��10-3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
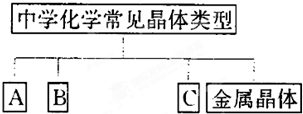
| A | B | C | ||
| ������� | �������� | |||
| ʵ���Ļ�ѧʽ | Na |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�����мס�����ƿ��ɫ��Һ������һƿ��ϡ���ᣬ��һƿ��̼������Һ��Ϊ�ⶨ�ס�����ƿ��Һ�ijɷּ����ʵ���Ũ�ȣ���������ʵ�飺
ȡ25mL����Һ�������л�����������Һ15mL�����ռ���224mL����״�������塣
ȡ15mL����Һ�������л����������Һ25mL�����ռ���112mL����״�������塣
��1���жϣ����� ��Һ������ ��Һ��
��2��ʵ������������Ӧ�����ӷ���ʽΪ�� ��
��3������Һ�����ʵ���Ũ��Ϊ ������Һ�����ʵ���Ũ��Ϊ ��
��4����n mL����Һ����������Һ����������ʵ�鷽ʽ���з�Ӧ���������������ΪVmL����״���£�����V��ȡֵ��Χ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������Ļ����ϣ��������֮������ϵ������֪ʶ������ѧϰ��ѧ����Ҫ��������ͼ����ѧ��ѧ������ѧ����֮������ϵ��
 |
��1�������� ����±�����ʵ��������Ҫ����д��
��ֻ����H��O��N��Si��SԪ���е�һ�ֻ���������ʣ���ÿ��Ԫ��ֻ�ܳ���һ�Σ�����������ຬһ��ԭ���ţ����������ʱ����ܹ��ش����⣨2�������⣨3����
A | B | C | ||
������� | �������� | |||
ʵ���Ļ�ѧʽ | Na |
��2��ȡ����������A��B��C���־�����ijһ��������ˮ��W��Һ��д�������ʵ���Ũ�ȵ����������������W��Һ��Ӧ�����ӷ���ʽ�������������������������� ��
��3��д�������������۵���ߵľ����������������Ʒ�Ӧ�Ļ�ѧ����ʽ�������������� ��
![]()
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com