
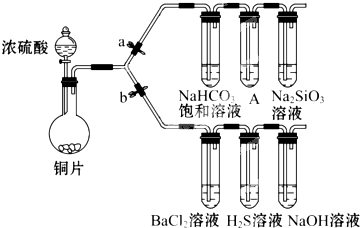
 ����Ҫ��������и�С��ʵ��Ŀ�ģ���a��b Ϊ���ɼУ����ȼ��̶�װ������ȥ��
����Ҫ��������и�С��ʵ��Ŀ�ģ���a��b Ϊ���ɼУ����ȼ��̶�װ������ȥ��| �μӵ���Һ | �ȡ�ˮ | ����ˮ |
| �����Ļ�ѧʽ |
| ||
| ||
| BaSO4 | BaSO3 |


 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
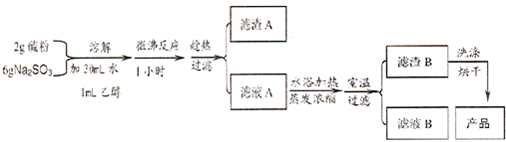
| �������� | ������ˮ���������Ҵ����۵� 48.2�棻�ڳ�ʪ�Ŀ������׳��� |
| ��ѧ���� | 43�����ϵĿ������绯�������ֽ� ��S2O32-+2H+=S��+SO2��+H2O �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ũ�Ⱦ�Ϊ0.1 mol/L��NH4Cl��Һ�Ͱ�ˮ�������Ϻ�c��NH4+��+c��NH3?H2O��=2c��Cl- �� |
| B��������ˮ�У�c��Cl- ����c��H+ ����c��OH- ����c��ClO- �� |
| C��pH=5.6��CH3COOH��CH3COONa�����Һ�У�c��CH3COO-����c��Na+����c��H+ ����c��OH- �� |
| D�������£�pH=2�������pH=12�İ�ˮ�������Ϻ�c��NH4+ ����c��Cl- ����c��OH- ����c��H+ �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���������ױ�������
���������ױ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
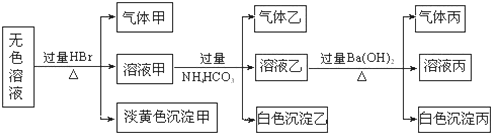
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
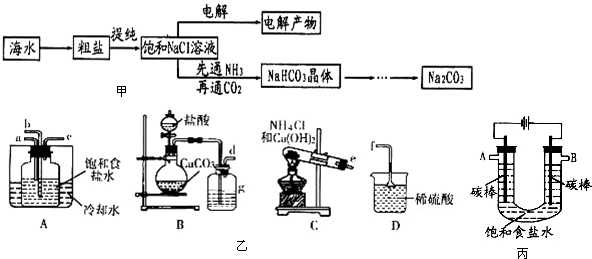
A��ʵ������װ��A��ȡ������ |
B����Bװ�����հ���������ֹ������ |
C����Cװ��ϡ��Ũ����C�� |
D����Dװ�ó�ȥCO2�е�HCl�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ���� | ��Ӧ����£� | ���� |
| a | 10mL5%H2O2��Һ+1mLH2O | 0.1gMnO2��ĩ |
| b | 10mL5%H2O2��Һ+1mLϡHCl | 0.1gMnO2��ĩ |
| c | 10mL5%H2O2��Һ+1mLϡNaOH��Һ | 0.1gMnO2��ĩ |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com