
��20�֣���1��ͬ���칹���������л���ѧ���Ƿdz��ձ�ģ������л��ﻥΪͬ���칹����� ��
��CH2��CHCH3 �� ��CH3CH2CH3 ��HC
��CH3CH2CH3 ��HC CCH3
CCH3
��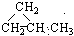 ��CH3CH��CHCH3
��CH3CH��CHCH3
д����������״ͬ���칹�弰���� ��
��2������ҹ�����������һ������ȼ�ϵ�أ�һ���缫ͨ���������һ���缫ͨ��������������صĵ�����Dz����� ��
�� ���壬���ڸ������ܴ���
���壬���ڸ������ܴ���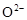 ���ش��������⣺���Զ��飨
���ش��������⣺���Զ��飨 ���������ͣ������طŵ�ʱ������Ӧ�Ļ�ѧ����ʽ��_________________________________________________________��
���������ͣ������طŵ�ʱ������Ӧ�Ļ�ѧ����ʽ��_________________________________________________________��
�������ص����������ķ�Ӧ��_________________������������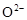 ���ƶ�������__________________ ��
���ƶ�������__________________ ��
��3����ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��
2CH3OH+3O2+4KOH 2K2CO3+6H2O

����ش𣺼׳��� װ�ã�B��ʯī���缫�������� ��
��д�����е缫��Ӧʽ�� ͨ��CH3OH �ĵ缫�ĵ缫��Ӧʽ�� ��
A��Fe���缫�ĵ缫��ӦʽΪ ��
���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ ��
�ܵ��ҳ���A��Fe��������������5.40gʱ���׳���������ת�Ƶ��� mol��
1���٢� �ݢ� CH2��CHCH2CH3 1����ϩ
CH2��C��CH3��2 2������ϩ����1�֣�
��2���� ����
����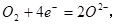 ������������1�֣���
������������1�֣���
��3����ԭ��أ�1�֣� ������1�֣� ��CH3OH-6e-+ 8OH- ==6H2O +CO32-
Ag++e��="=Ag" ��1�֣�
��4AgNO3+2H2O 4Ag+O2��+4HNO3 ��0.05 ������ÿ��2�֣�
��������
�����������1��ͬ���칹�壺����ʽ��ͬ���ṹ��ͬ�Ļ����
��2����ȼ�ϵ���ܵķ���ʽΪ ���ָ����ʻ��ϼ۵����ۿ���֪����O��0�۽��͵�-2�ۣ�������ԭ��Ӧ����Ϊ������O-2���������������������ʻ�������
���ָ����ʻ��ϼ۵����ۿ���֪����O��0�۽��͵�-2�ۣ�������ԭ��Ӧ����Ϊ������O-2���������������������ʻ�������
��3���ټ׳���һ��ԭ��أ��ҳ�Ϊһ�����ء�ͨ�������ĵ缫������ԭ��Ӧ��Ϊԭ��ص�������������������B�缫Ϊ������
���Ҵ��ڼ�����Һ�зŵ磬�缫����ʽΪCH3OH �� 6e-+ 8OH- ==6H2O +CO32- ��A�缫Ϊ���ص�������������ԭ��Ӧ���ʵ缫����ʽΪAg++e��==Ag��
��A�缫���ӵ�����������������4AgNO3+2H2O 4Ag+O2��+4HNO3 ת�Ƶ�����
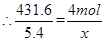
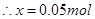
��ת��0.05mol�ĵ��ӡ�
���㣺ͬ���칹�塢ԭ���ԭ���͵���ԭ��
���������⿼����ͬ���칹��ĸ���л��������������ԭ��ء����صĹ���ԭ�����Ѷ��еȡ����ڸ߿��������ͣ���ע�⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ���ǶԱ��Ľṹ�����ʵ���ʶ������һ�������Ĺ��̣�
���ǶԱ��Ľṹ�����ʵ���ʶ������һ�������Ĺ��̣� +Br
+Br| ���� |
 -Br+HBr
-Br+HBr +Br
+Br| ���� |
 -Br+HBr
-Br+HBr�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ��ͷ�н�ɽ��ѧ�߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��20�֣���1��ͬ���칹���������л���ѧ���Ƿdz��ձ�ģ������л��ﻥΪͬ���칹����� ��
��CH2��CHCH3 �� ��CH3CH2CH3 ��HC
��CH3CH2CH3 ��HC CCH3
CCH3
�� ��CH3CH��CHCH3
��CH3CH��CHCH3
д����������״ͬ���칹�弰���� ��
��2������ҹ�����������һ������ȼ�ϵ�أ�һ���缫ͨ���������һ���缫ͨ��������������صĵ�����Dz����� ��
�� ���壬���ڸ������ܴ���
���壬���ڸ������ܴ��� ���ش��������⣺���Զ��飨
���ش��������⣺���Զ��飨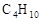 ���������ͣ������طŵ�ʱ������Ӧ�Ļ�ѧ����ʽ��_________________________________________________________��
���������ͣ������طŵ�ʱ������Ӧ�Ļ�ѧ����ʽ��_________________________________________________________��
�������ص����������ķ�Ӧ��_________________������������ ���ƶ�������__________________ ��
���ƶ�������__________________ ��
��3����ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��
2CH3OH+3O2+4KOH 2K2CO3+6H2O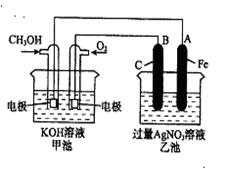
����ش𣺼׳��� װ�ã�B��ʯī���缫�������� ��
��д�����е缫��Ӧʽ�� ͨ��CH3OH �ĵ缫�ĵ缫��Ӧʽ�� ��
A��Fe���缫�ĵ缫��ӦʽΪ ��
���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ ��
�ܵ��ҳ���A��Fe��������������5.40gʱ���׳���������ת�Ƶ��� mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��ͬ���칹���������л���ѧ���Ƿdz��ձ�ģ������л��ﻥΪͬ���칹����� ��
��CH2��CHCH3 ��![]() ��CH3CH2CH3 ��HC
��CH3CH2CH3 ��HC![]() CCH3
CCH3
��![]() ��CH3CH��CHCH3
��CH3CH��CHCH3
д����������״ͬ���칹�弰���� ��
��2������ҹ�����������һ������ȼ�ϵ�أ�һ���缫ͨ���������һ���缫ͨ��������������صĵ�����Dz�����![]() ��
��![]() ���壬���ڸ������ܴ���
���壬���ڸ������ܴ���![]() ���ش��������⣺���Զ��飨
���ش��������⣺���Զ��飨![]() ���������ͣ������طŵ�ʱ������Ӧ�Ļ�ѧ����ʽ��_________________________________________________________��
���������ͣ������طŵ�ʱ������Ӧ�Ļ�ѧ����ʽ��_________________________________________________________��
�������ص����������ķ�Ӧ��_________________������������![]() ���ƶ�������__________________ ��
���ƶ�������__________________ ��
��3����ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��
2CH3OH+3O2+4KOH 2K2CO3+6H2O
 ����ش𣺼׳��� װ�ã�B��ʯī���缫�������� ��
����ش𣺼׳��� װ�ã�B��ʯī���缫�������� ��
��д�����е缫��Ӧʽ�� ͨ��CH3OH �ĵ缫�ĵ缫��Ӧʽ�� ��
A��Fe���缫�ĵ缫��ӦʽΪ ��
���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ ��
�ܵ��ҳ���A��Fe��������������5.40gʱ���׳���������ת�Ƶ��� mol��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com