



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
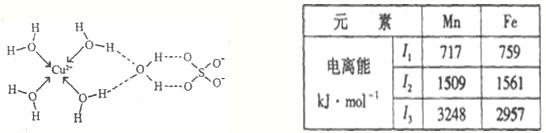
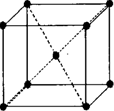 ��2011?�����ģ��[ѡ�����ʽṹ������]��֪A��B��C��D��E����Ԫ�صĺ˵������������EΪ��������Ԫ���⣬����Ƕ�����Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AԪ���������������ڲ��������2����BԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��EԪ�ص�+3�����ӵ�3d�ܼ�Ϊ�����״̬��������ʱ��ABCDE��Ӧ��Ԫ�ط��ű�ʾ��
��2011?�����ģ��[ѡ�����ʽṹ������]��֪A��B��C��D��E����Ԫ�صĺ˵������������EΪ��������Ԫ���⣬����Ƕ�����Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AԪ���������������ڲ��������2����BԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��EԪ�ص�+3�����ӵ�3d�ܼ�Ϊ�����״̬��������ʱ��ABCDE��Ӧ��Ԫ�ط��ű�ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
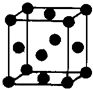 ��2012?�人ģ�⣩[��ѧһѡ�� 3�����ʽṹ������]��U��V��W��X��Y��Z ����ǰ������Ԫ�أ�ԭ���������������������Ϣ���±���
��2012?�人ģ�⣩[��ѧһѡ�� 3�����ʽṹ������]��U��V��W��X��Y��Z ����ǰ������Ԫ�أ�ԭ���������������������Ϣ���±���| Ԫ�ر�� | �����Ϣ�� |
| U | ���������������������ֱ�����ԭ��������� |
| V | ��̬ʱ�����ӷֲ��������ܼ��ϣ��Ҹ��ܼ��е�������� |
| W | ��̬ʱ��2p ������ڰ����״̬ |
| X | ��WԪ�ش���ͬһ���ڣ���X�ĵ�һ������С��W�ĵ�һ���� |
| Y | �ǵ�������Ԫ����δ�ɶԵ���������Ԫ�� |
| Z | Z ��һ�ֺ��ص�������Ϊ65��������Ϊ 36 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com