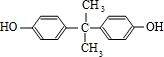
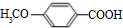
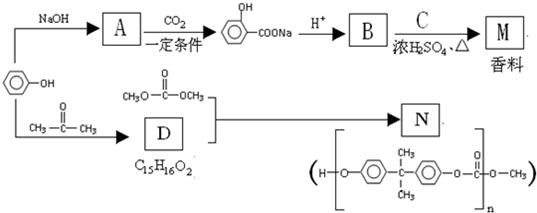
��Ҫ�Ļ���ԭ��M��C
5H
8O
4�����������ζ������������ͼ��ʾ�������̺ϳɣ�
��֪��A��B��Ϊͬ���칹�壻X���ܶ�����ͬ������H2�ܶȵ�21����D��F��M������NaHCO3?��Һ��Ӧ�������壮
��1��A��ϵͳ������������Ϊ
2-����
2-����
��
��2��X�Ľṹ��ʽ��
CH2=CHCH3
CH2=CHCH3
��E�Ľṹ��ʽ��
ClCH2COOH
ClCH2COOH
��
��3���١��ķ�Ӧ������ȡ����Ӧ����
�ܢݢ�
�ܢݢ�
��
��4��д������ʽ��B��C
2CH
3CH
2CH
2OH+O
22CH
3CH
2CHO+2H
2O
2CH
3CH
2CH
2OH+O
22CH
3CH
2CHO+2H
2O
��D+F��M
CH
3CH
2COOH+HOCH
2COOH
CH
3CH
2COOCH
2COOH+H
2O
CH
3CH
2COOH+HOCH
2COOH
CH
3CH
2COOCH
2COOH+H
2O
��
��5��M��ͬ���칹���кܶ࣬����һ��ͬ���칹��ֻ����һ�ֹ����ţ������Ժͼ��������¶���ˮ�����������л����ͬ���칹��Ľṹ��ʽ��
CH3OOCCH2COOCH3��HCOOCH2CH2CH2OOCH��CH3COOCH2OOCCH3
CH3OOCCH2COOCH3��HCOOCH2CH2CH2OOCH��CH3COOCH2OOCCH3
��









 �Ļ�ѧ����ʽ��
�Ļ�ѧ����ʽ��




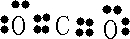
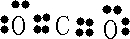
 H++Cl-+HClO
H++Cl-+HClO H++Cl-+HClO
H++Cl-+HClO
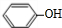 +NaOH��
+NaOH��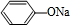 +H2O
+H2O +NaOH��
+NaOH�� +H2O
+H2O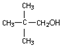

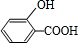 +
+
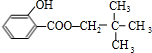 +H2O
+H2O +
+
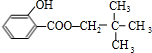 +H2O
+H2O